Although brain-dead organ donors have routinely been maintained on life-support until recipients of their donor organs could be identified, UAB is now the first to publish the results of this novel human preclinical model to test important safety and feasibility issues in a 57-year-old male brain-dead organ recipient.
This is required because the extensive tests of transplanted humanized pig kidneys into non-human primates cannot answer critical questions due to biologic differences between non-human primates and humans. These gaps in knowledge concerning multiple risks must be filled before developing the first clinical trial in living humans.
One question is: Can a kidney from a pig tolerate an adult human environment? Blood pressure is one hurdle because non-human primates and pigs have lower mean arterial blood pressures than adult humans. Without the human preclinical model, surgeons could not be sure that vascular integrity would hold up after the transplant. Equally important, the UAB researchers say, was the relative hemodynamic stability of the decedent upon reperfusion, indicating that washout of inflammatory mediators from the xenograft did not provoke cardiovascular collapse.
The experiment also tested whether life-threatening complications might occur during surgery.
“By actually doing this transplant,” said transplant surgeon Jayme Locke, M.D., “we were able to show that you could take a kidney from a pig that had been genetically modified put that into an adult brain-dead human and actually have it hold its integrity, so it perfused normally, just like a human allograft. The vascular anastomoses stayed intact, and we didn’t have any major bleeding episodes, all things that are important to establish in a preclinical human model before we take this into living humans.”
Locke is a professor in the UAB Marnix E. Heersink School of Medicine Department of Surgery Division of Transplantation.
Immune rejection
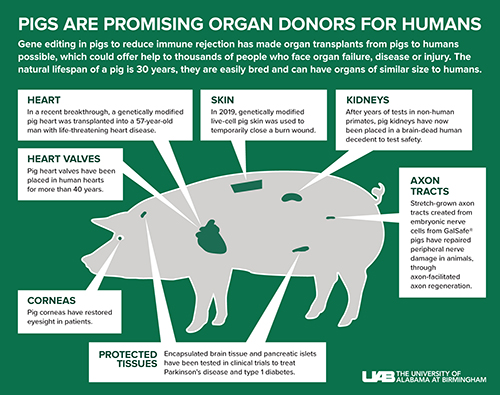
Gene editing in pigs to reduce immune rejection has made organ transplants from pigs to humans possible, which could offer help to thousands of people who face organ failure, disease or injury. The natural lifespan of a pig is 30 years, they are easily bred and can have organs of similar size to humans.
Non-human primate research cannot fully answer whether the humanized pig kidney will avoid hyperacute rejection in humans. Only a human xenotransplantation experience can fully test this.
Hyperacute rejection occurs within minutes if the recipient’s immune system recognizes a donor organ as foreign, says UAB transplant surgeon Paige Porrett, M.D., Ph.D., associate professor in the UAB Department of Surgery’s Division of Transplantation and director of Clinical Translational Research for the Comprehensive Transplant Institute. When the UAB team reperfused the transplanted pig kidneys last fall, “We watched with bated breath, for seconds, for minutes.”
“The kidney turned beautiful and pink,” Locke said, “and within 23 minutes, it started making urine.” The kidneys remained viable until the experiment was ended after 77 hours.
Before any human kidney allotransplantation at UAB — that is, a transplant from a living or brain-dead human donor to an end-stage kidney disease recipient — the UAB Histocompatibility and Immunogenetics Laboratory does a crossmatch. This immune test reveals whether the donor organ is compatible or incompatible with the recipient.
For last fall’s pig kidney xenotransplantation, the lab had to develop novel crossmatch test controls for pig-to-human compatibility, using a shotgun approach. By screening hundreds of stored human recipient sera, the laboratory team was able to establish positive and negative controls, the first time this has been accomplished. The positive control serum is human serum containing immunoglobulin G known to react with pig cells. This monumental effort was led by Vera Hauptfeld-Dolejsek, Ph.D., F(ACHI), lab director and a professor in the UAB Department of Surgery’s Division of Transplantation, and Julie Houp, lab associate director
This crossmatch test screens for the presence of preformed antibodies in the recipient that react with antigens in the donor pig that would cause a hyperacute kidney rejection. Blood serum from the recipient is mixed with lymphocytes from the proposed donor, and then screened in a flow cytometry crossmatch assay, the gold standard. The test takes four to six hours.
“The development of this novel assay was absolutely critical for us to be able to use xenotransplantation widely for a living human population with end-stage kidney disease,” Locke said. “Now we have evidence that this crossmatch we developed is very accurate and can predict compatibility between the pig xenograft and the human recipient.”
One big unknown remains — if there is reactivity against the pig organ, what exact antigens do the sera antibodies recognize? More work must be done to refine the pig-to-human crossmatch test.
For the UAB xenotransplant team, the crossmatch test performed before surgery showed compatibility between the pig kidneys and the decedent recipient. However, only the actual operation could confirm compatibility.
An important contribution of this research was the monitoring of the health of the kidneys with daily biopsies by the UAB Department of Pathology. These showed the formation of microscopic blood clots of unclear cause and significance. UAB’s team is performing additional investigations to better understand these findings.
Click image to enlarge
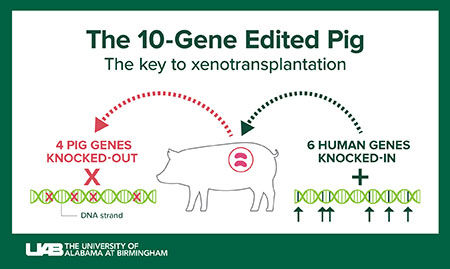
Graphic: Jody PotterThe 10-gene, humanized pig
Revivicor, a subsidiary of United Therapeutics Corporation, provided the genetically modified pig that was the source of the investigational xenotransplant kidneys called UKidney™. Efforts by many researchers to genetically modify pigs through genetic changes that reduce the chance of rejection began in the early 2000s. Since the ancestors of pigs and humans diverged 80 million years ago, the genetic changes in the pig are necessary to overcome biologic barriers created by 80 million years of evolution.
Ten genes have been changed in the donor pig. Four are disabled pig genes, also known as knockouts. Six are human genes that have been cloned into the pigs, also known as knock-ins.
Three knockouts have disabled genes for enzymes that add carbohydrate structures to pig cells that would cause hyperacute rejection. The pig genes are GGTA1, β4GalNT2 and CMAH. The fourth knockout is the pig hormone growth receptor gene, to prevent the kidneys from growing too large in the recipient.
The human genes added to the pig are meant to help prevent rejection, whether immediate hyperacute rejection or even slower types of rejection that can occur up to months or years after transplantation.
Two knock-in genes — human DAF, also known as CD55, and human CD46 — are complement inhibitor genes. Complement is part of the immune system that enhances the ability of antibodies to attack microbes, damaged cells or, in xenotransplantation, foreign cells. Considerable evidence from xenotransplantation into non-human primates shows the beneficial effect of these two complement regulatory genes.
Two knock-in genes — human TBM, or thrombomodulin, and human EPCR, or endothelial C receptor — are blood coagulation regulatory proteins. These have shown benefits to prevent formation of microscopic blood clots, called thrombotic microangiopathy, in the graft. Thrombotic microangiopathy can lead to consumptive coagulopathy, the deadly formation of blood clots throughout the body. These coagulation regulatory proteins have been shown to increase xenotransplant organ survival in non-human primates.
The final two knock-in genes — human hemeoxygenase-1, or HO1, and CD47 — are immunomodulatory genes meant to reduce inflammation in the xenograft. HO1 has strong anti-oxidative, anti-apoptotic and anti-inflammatory effects. Apoptosis is the programmed cell death that occurs when a cell is damaged or infected. CD47 acts to suppress the activation of phagocytic macrophages and the infiltration of T cells, two arms of the body’s white blood cell immune response. Although white blood cells function to protect against illness and disease, these cells also participate in injury of organ grafts.

Click image to enlarge
Graphic: Jody PotterLimits for the preclinical human model
The preclinical human model helps answer important questions about the feasibility and safety of xenotransplantation. Researchers can use this information to design a clinical trial for xenotransplantation into living humans. However, some questions are difficult to answer through this model.
One is measuring the basic functions of the kidney, because a transplanted kidney is exquisitely sensitive to the environment where it is placed. The distressed physiology seen in the context of brain death is a hostile environment for transplantation, which delays recovery of the stressed organ. This limits assessment of normal kidney functions, which include the regulation of extracellular blood fluid volume, osmolarity, ion concentration and pH; the excretion of wastes and toxins; and production of hormones.
“Trying to ascertain function in the face of brain death is always going to be challenging,” Locke said. “Ultimately, we will need to move into a Phase I clinical trial in which we transplant these kidneys into a living human being where the environment is more favorable for kidney recovery.”
In the UAB study last fall, one kidney produced urine approximately 23 minutes after reperfusion in the decedent, while the other kidney had scant urine production. Despite urine production, the blood serum level of creatinine, a waste product normally removed by healthy kidneys, did not drop. It’s not clear yet to the UAB team why there was a difference in urine output between the kidneys. It is also not yet clear what factors may have impacted the inability of the kidneys to clear creatinine from the blood.
Although the UAB team did not see traditional signs of organ transplant rejection in the pig kidneys, slower types of rejection are difficult to measure in the preclinical human model because of the limited duration of the experiment. For example, UAB’s xenotransplantation experiment was ended after three days, too short a period to measure signs of acute rejection that can take up to weeks or months to appear. Yet the model is a bridge to solving a crisis — the shortage of kidneys for transplant. There is a clear and urgent need for xenotransplantation, physicians say.
Click image to enlarge
Graphic: Jody Potter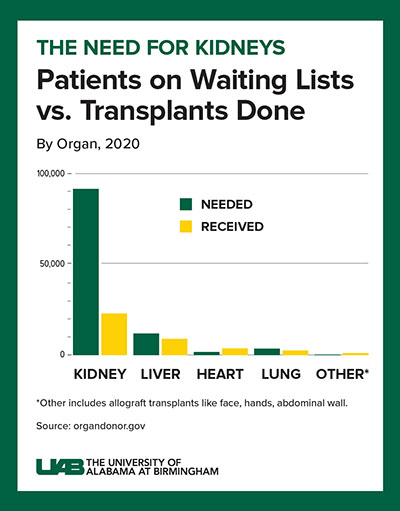
Click image to enlarge
Graphic: Jody PotterXenotransplants would be lifesaving
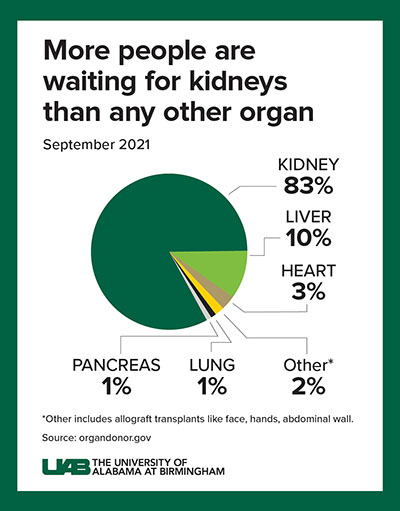
Decades of human-to-human kidney transplants have shown that a kidney recipient has a greater longevity and quality of life than a person on chronic dialysis. About 5 to 15 percent of dialysis patients die yearly, and the eight-year survival rate is only about 35 percent.
A kidney transplant, in contrast, works more than 95 percent of the time, curing the end-stage kidney disease. However, fewer than 25,000 kidney transplants are performed each year, and more than 90,000 people are on the transplant waiting list. More than 800,000 Americans are living with kidney failure, and most never make it to the waiting list.
This shows a radical solution is needed for the organ supply crisis, Locke says. If many people are compatible for a xenograft from a pig, that — paired with human-to-human allotransplantation — could potentially wipe out the kidney transplant waiting list.
“The concept of being able to have an organ waiting on the shelf, waiting for the person who needs it, is just remarkable to think about, and exciting for that person,” Locke said. “I feel really privileged to be just a tiny part of a really big puzzle that people have been working on for many years.”
Advancing xenotransplantation to the clinic
The xenotransplantation program at UAB began six years ago, with the goal to advance xenotransplantation into the clinical realm. One piece of the effort was building a pathogen-free animal facility, with a surgical suite, committed only to human xenotransplantation.
Pigs living there are kept free of specified infectious agents like pig cytomegalovirus through rigorous practices of veterinary care. In addition, through herd husbandry, the porcine endogenous retrovirus C (PERV-C) has been bred out of the pigs, helping to prevent the chance of PERV transmission to human organ recipients. The donor pigs are tested every three months, by the University of Minnesota Veterinary Diagnostic Laboratory, for 13 pig viruses and one mycoplasma.
At UAB last fall, the recipient of the xenograft kidney was closely monitored for any infection by viruses that are in the pig genomes. Blood samples from the xenotransplant recipient were tested daily for the presence of pig endogenous retroviruses; all samples were negative. Also, there was no sign of pig-derived cells in the blood of the recipient.
Another key design of UAB’s first clinical grade pig-to-human kidney transplant was making it mirror — as much as possible — every step of a normal kidney allograft between humans. This included Institutional Review Board approval; a crossmatch before starting the operations; using the standard procedures of allotransplants to remove, preserve, transport and transplant the kidneys; and giving the standard immunosuppression therapy to the decedent recipient. The only significant differences were the source of the kidney and extra treatment needed to maintain metabolic homeostasis in the decedent recipient.
This mimicking of a normal allotransplantation was a test of the UAB xenotransplantation program infrastructure, and it lays the groundwork for a future clinical trial. “In order to get the FDA’s approval, we have to be able to demonstrate to them that we can carry out xenotransplantation in the same safe and feasible manner that we do every day when we do an allotransplant,” Locke said. “The only thing different is that the kidney came from a pig.”
Click image to enlarge
Graphic: Jody PotterDecades of slow progress
History shows that improvement in organ transplantation is slow and incremental. One commentator has described it as a story of barriers, and how modern medicine has overcome those barriers. Another called it the history of many unsuccessful efforts and setbacks.
More than five decades have passed since a Tulane University surgeon transplanted chimpanzee kidneys into 13 end-stage kidney disease patients in 1963-64. Those experimental recipients had little alternative other than death because chronic dialysis was not then available. Despite the close evolutionary relationship between chimpanzees and humans, no patient survived this desperate surgery for more than nine months, and nearly all died within weeks.
Yet the tantalizing promise of kidney transplants had been established in 1954 with a successful human-to-human transplant between identical-twin brothers. That transplanted kidney functioned for eight years.
In 1962 came the first kidney transplant between genetically nonrelated patients, using immunosuppression. Improved understanding of the immune system and improved management of immune rejection have greatly advanced the success of human kidney allotransplantation. UAB’s first kidney transplant came in 1968.
The proposal that pigs could be a better source of xenografts because their organs are closer in size to human organs — in contrast to the smaller size of organs from non-human primates — came in the 1980s. The use of pig organs for transplantation to humans appears closer now, greatly aided by sophisticated alteration of the pig genome. Some questions still remain, but the need has never been greater.
At UAB, Locke holds the Arnold G. Diethelm Endowed Chair in Transplantation Surgery and is director of the Division of Transplantation at UAB. She also is the director of the UAB Comprehensive Transplant Institute. Also at UAB, Porrett holds the Vera Hauptfeld-Dolejsek, Ph.D., Endowed Professorship in Transplant Immunology.