 The UAB Departments of Radiation Oncology and Cardiology are looking for patients with ventricular tachycardia (VT) not controlled by medications and catheter ablation.
The UAB Departments of Radiation Oncology and Cardiology are looking for patients with ventricular tachycardia (VT) not controlled by medications and catheter ablation.
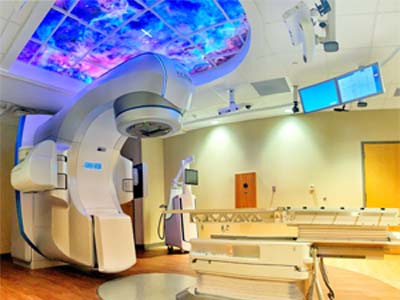
The trial will allow patients suffering from VT despite standard of care regimens to undergo non-invasive cardiac radioablation (CRA; a type of stereotactic body radiotherapy) to the area of the heart responsible for their symptoms. Stereotactic body radiotherapy is delivered with a device called the linear accelerator, which is primarily used to treat patients with cancer.
UAB is one of the first centers in the U.S. to obtain a Food and Drug Administration (FDA) investigational device exemption for use of the linear accelerator in this capacity on a phase I/II trial.
The study is being conducted in collaboration with the Washington University School of Medicine in St. Louis, a center which pioneered this promising technique in the U.S.
For more information on the RAD 1901 trial, please contact Adelyn Gillon, clinical research coordinator, at 205.975.3019 or agillon@uabmc.edu.