 Research in animal models shows better formation of the fistulas, which are a lifeline for kidney failure patients as the connection site to dialysis machines.More than 600,000 people in the United States have end-stage kidney disease, and about 450,000 are kept alive through chronic hemodialysis to remove waste products from their blood.
Research in animal models shows better formation of the fistulas, which are a lifeline for kidney failure patients as the connection site to dialysis machines.More than 600,000 people in the United States have end-stage kidney disease, and about 450,000 are kept alive through chronic hemodialysis to remove waste products from their blood.
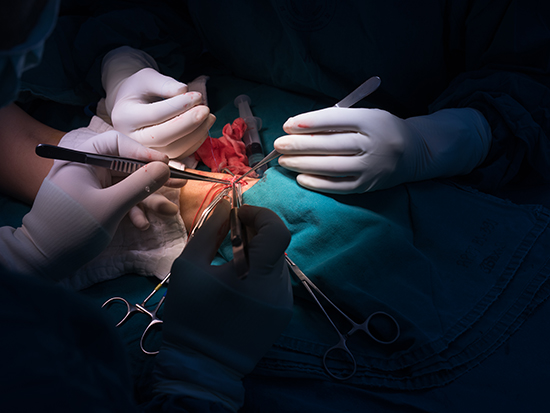
This requires durable access to a blood vessel site where the dialysis machine can remove and return blood. Such access is created when a surgeon attaches a vein to an artery in the patient. The pressure of the arterial blood forces the vein to expand. After this arteriovenous fistula, or AVF, develops and matures, that enlarged space becomes the lifeline for the hemodialysis patient.
Yet up to 60 percent of AVFs fail to develop adequately, due to smooth muscle cell hyperplasia, which is overgrowth inside the vessel, and inadequate expansion of the vein. This leads to illness and death in dialysis patients, and the treatment of vascular-access dysfunction costs more than $2 billion a year.
An experimental gel may reduce those failures.
Researchers at the University of Alabama at Birmingham and Endomimetics LLC are working to develop the first effective therapy to promote healthy AVF maturation. They recently reported preclinical progress in the journal Biomaterials, showing that an experimental gel helped create a fully developed AVF in rodents, along with significantly improved vascular access.
The UAB and Endomimetics researchers first showed that nitric oxide, or NO, plays an important regulatory role in AVF development. Mice that overexpressed endothelial nitric oxide synthase — an enzyme that synthesizes NO — had reduced intimal hyperplasia development and vein narrowing in their AVFs, compared to control mice. Thus, the researchers reasoned that finding a way to release NO at a newly formed AVF might support AVF development and maturation.
They developed a self-assembling, nanomatrix gel capable of releasing a burst of NO in the first 24 hours, followed by sustained NO release for four weeks.
When the gel was applied to a newly joined artery-vein meant to create an AVF in rats, the gel persisted at that site, and the rats showed a more than 70 percent reduction of intimal hyperplasia formation, as well as an improvement in vein diameter and a smoother blood flow, compared to controls.
“Therefore,” the researchers concluded, “direct application of the NO releasing nanomatrix gel to the AVF anastomosis immediately following AVF creation may enhance AVF development, thereby providing long-term and durable vascular access for hemodialysis.”
 (Standing) Brigitta Brott, M.D., Joseph Garner, Ph.D., Ho-Wook Jun, Ph.D., (seated) Timmy Lee, M.D., and Patrick Hwang, Ph.D.Joseph Garner, Ph.D., CEO of Endomimetics, says showing that the AVF Gel substantially increases the success rate of AVF development could result in dramatic improvement to the quality of life of patients, as well as a significant decrease in the cost of creating AVFs.
(Standing) Brigitta Brott, M.D., Joseph Garner, Ph.D., Ho-Wook Jun, Ph.D., (seated) Timmy Lee, M.D., and Patrick Hwang, Ph.D.Joseph Garner, Ph.D., CEO of Endomimetics, says showing that the AVF Gel substantially increases the success rate of AVF development could result in dramatic improvement to the quality of life of patients, as well as a significant decrease in the cost of creating AVFs.
Use of the gel to promote development of an AVF, he says, has been supported by National Institutes of Health Small Business Innovative Research, or SBIR, grants totaling more than $4.5 million. The company has completed SBIR Phase I and II grants, showing that the AVF Gel significantly improved vascular access, via a fully developed AVF, in small and large animal models. A recently awarded SBIR Phase IIB grant will support the large-scale manufacturing and safety studies required to pursue United States Food and Drug Administration approval of the gel as an Investigational New Drug.
Co-founders of Endomimetics are Ho-Wook Jun, Ph.D., professor in the UAB Department of Biomedical Engineering, and Brigitta Brott, M.D., an interventional cardiologist and professor in the UAB Department of Medicine Division of Cardiovascular Disease. At Endomimetics, Jun is chief scientific officer, and Brott is chief medical officer.
Co-senior authors in the Biomaterials study, “Nitric oxide releasing nanomatrix gel treatment inhibits venous intimal hyperplasia and improves vascular remodeling in a rodent arteriovenous fistula,” are Jun and Timmy Lee, M.D., UAB Department of Medicine Division of Nephrology. Co-first authors are Maheshika Somarathna, UAB Division of Nephrology, and Patrick TJ. Hwang, Ph.D., Endomimetics.
Other co-authors besides Brott are Reid C. Millican and Jennifer A. Sherwood, Endomimetics; Grant C. Alexander, UAB Department of Biomedical Engineering; Tatyana Isayeva-Waldrop, UAB Division of Nephrology; Isabelle Falzon, Hannah Northrup, Yan-Ting Shiu and Chris J. Stubben, University of Utah; and John Totenhagen, UAB Department of Radiology.
Support came from NIH grants DK109789, DK109789-03 and -04, DK100505, DK121227, HL139692, HL153244 and HL125391; Department of Veterans Affairs Merit Awards I01BX003387 and I01BX004133; and Alabama Research and Development Enhancement Fund 1ARDEF22 09.
UAB Biomedical Engineering is a joint department of the UAB Marnix E. Heersink School of Medicine and the UAB School of Engineering. Medicine and Radiology are departments in the UAB Heersink School of Medicine.