 Melissa Harris's research points to a new paradigm for hair graying. “We thought that once you go gray the stem cells are all lost — there’s no going back," Harris said. "But presumably they can be reactivated."
Melissa Harris's research points to a new paradigm for hair graying. “We thought that once you go gray the stem cells are all lost — there’s no going back," Harris said. "But presumably they can be reactivated."
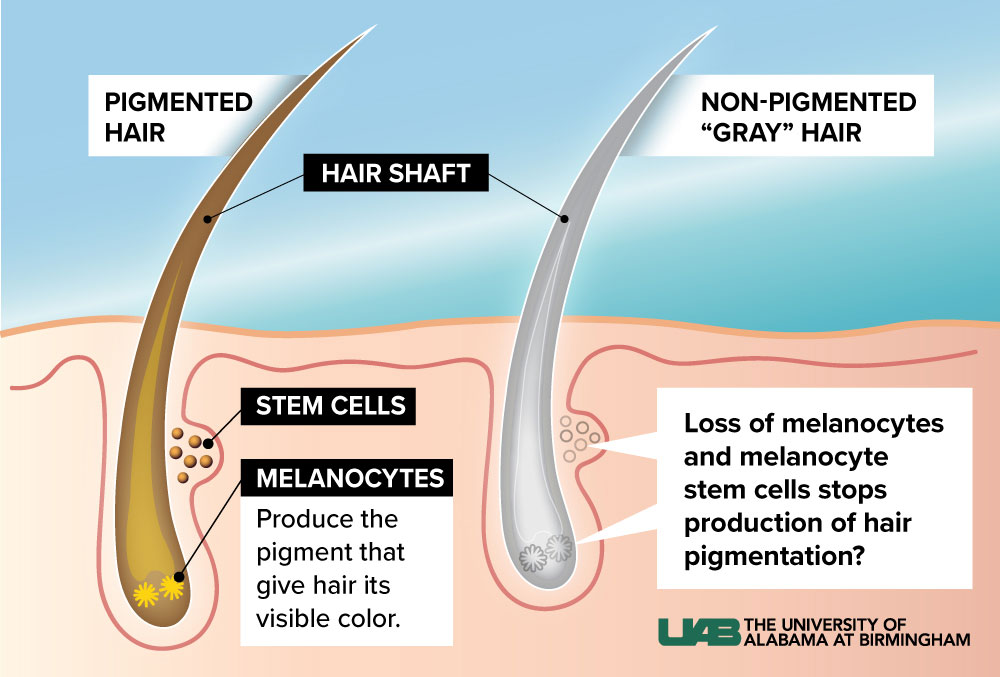
Molecular biology is not usually the kind of science you can do with the naked eye. Sure enough, Melissa Harris, Ph.D., runs a lab that leans into CRISPR gene-editing tools, single-cell sequencing studies and network-analysis algorithms. But all she needs is a glance to diagnose the state of your melanocyte stem cells.
That’s the beauty of gray hair as a model for aging: If your hair is all one color, the melanocyte stem cells are doing fine. Wherever there are gray hairs mixed in, however, something has gone wrong.Are you getting old? Could be. Is this an inevitable part of the aging process? Not so fast. Harris, an assistant professor in the Department of Biology, has spent the past decade uncovering evidence that age isn’t the only reason melanocyte stem cells fail.
Now she is putting her catalog of gray-haired mouse models to work on a new task: demonstrating that there may be a way to bring those cells — and their original colored pigments — back from the dead. Harris’s lab is working with a biotech startup to study an experimental compound that appears to restore hair color for the long term in mice.
Hair: the perfect model
Recoloring hair is not Harris’s end goal. Sure, her work has applications to pigmentary disorders like the autoimmune disease vitiligo and to the most common skin cancer, melanoma, which is cancer of the melanocyte cells. But ultimately, she wants to understand why somatic stem cells — which are found in muscles, bone and organs throughout the body and are crucial for tissue regeneration, immune defense, wound healing and, yes, hair color — falter as we age. “To treat or prevent aging we need to be more specific — we’ll need personalized therapeutics” based on individual genetic or molecular factors, Harris said. That requires learning more about aging-related processes, including the mechanisms that disrupt the life cycles of the body’s stem cells.
Most of these stem cell populations are notoriously hard to work with in the lab; it is difficult to manipulate the genes involved in stem cell function without catastrophic results. But melanocyte stem cells are an exception.
“Does hair graying cause you to die?” Harris said. “No, you can watch melanocyte stem cells from birth to the end.” The same can’t be said for hematopoetic stem cells, which pump out new red blood cells in the bone marrow, for example. “You can’t live long without them,” Harris said.
 Microscope images demonstrate progressive loss of pigment in individual strands of hair. Image courtesy Melissa Harris
Microscope images demonstrate progressive loss of pigment in individual strands of hair. Image courtesy Melissa Harris
‘An average level of vanity’
“Everyone gets gray hair,” Harris said. But hair-graying research has a bad reputation that keeps many investigators away. “It is considered vanity science,” Harris said. As she likes to emphasize during her talks, however, “I am not an abnormally vain person,” she insists. “My lab has picked the model that is the most appropriate method to investigate what happens to stem cells as we age.” The work has earned her a prestigious K99/R00 Pathway to Independence grant from the NIH’s National Institute of Aging, and some notoriety in the aging field. Professional colleagues call her “the gray-hair lady,” Harris said.
The Harris lab already had explored immune activity in hair graying. Their 2018 paper in the journal PLOS Biology demonstrated that MITF, a protein that is the master regulator turning on pigmentation genes, also represses the innate immune system. When Harris challenged MITF-deficient mice with a virus, the melanocyte stem cells suffered. “When we gave the virus, they got gray hair,” she said. “And that didn’t happen in the wildtype [normal] mice.”
The research drew attention from media outlets around the world, with headlines such as “Study: This is why your hair turns gray” (Atlanta Journal-Constitution), “Grey hair may have roots in immune system” (Nature) and “Overactive immune response linked to hair graying” (Reuters).
“Perhaps, in an individual who is healthy yet predisposed for gray hair,” because they naturally produce less MITF, “getting an everyday viral infection is just enough to cause the decline of their melanocytes and melanocyte stem cells, leading to premature gray hair,” Harris told UAB News. The study also could “explain stories of a brother or uncle who got gray hair out of the blue or in early life,” she noted. “This could be one mechanism potentially, although it has not been investigated in humans yet.”
Being known for a niche interest has its advantages. “People come to me all the time when they accidentally discover gray-hair mouse models.” Or when they have the opposite problem. That’s what happened this past year when Harris got a query from a small biotech startup. They had an experimental compound in development to regrow hair. It seemed to affect pigmentation, too, and the company wanted to know more about the mechanism. Would Harris test it in her mouse models?
“I was skeptical,” Harris said. “But I said, ‘Send me the compound and we’ll do a small trial.’ Ends up it looks great.” When Harris treats gray-haired mice with the compound, she sees hair repigmentation. “And we can take these same mice, pluck the hair and when new hairs grow out they retain the higher level of pigmentation, suggesting this is permanent,” she said. “This compound is reprogramming the stem cells, taking them to a younger state, allowing them to start up again.”
Back from the dead?
Harris and one of her doctoral students, Joseph Palmer, suspect that one way in which melanocyte stem cells might be affected with age has to do with a phenomenon called the “quiescence program.” Most types of stem cells are needed only sporadically, to replace a specialized population of cells — such as the melanocyte stem cells, which are called on every seven years or so, when a new hair grows in. They spend the rest of their time sitting around in a dormant, or quiescent, twilight form. Harris calls it a “sleepy state.”
| “To treat or prevent aging we need to be more specific — we’ll need personalized therapeutics” based on individual genetic or molecular factors. That requires learning more about aging-related processes, including the mechanisms that disrupt the life cycles of the body’s stem cells. |
Quiescence helps preserve stem cells during life, lowering their metabolic rate and proliferative activity. Quiescence has been noted in muscle stem cells, hematopoietic stem cells, hair follicle stem cells, neural stem cells and intestinal stem cells, according to a 2019 paper by German researchers in the journal Trends in Cell Biology.
Other researchers have found that the timing of hair cycles “changes as you get older, with longer, more extended sleepy stages,” Harris said. “Joseph wondered what happens when stem cells are sleepy for longer periods of time. That’s one question we’re addressing.”
‘Presumably they can be reactivated’
The immune angle had turned up a few months earlier in an unexpected way. Spanish physicians writing in JAMA Dermatology noted a strange side-effect in more than a dozen patients receiving anti-programmed death receptor ligand 1 (anti-PD-L1) immunotherapy as treatment for lung cancer: hair repigmentation. PD-L1 is a protein that suppresses the immune system. Blocking it allows for a vigorous immune response against cancer cells.
“That suggested that some melanocyte stem cells are retained in gray hairs,” Harris said. “We thought that once you go gray the stem cells are all lost — there’s no going back. But presumably they can be reactivated.”
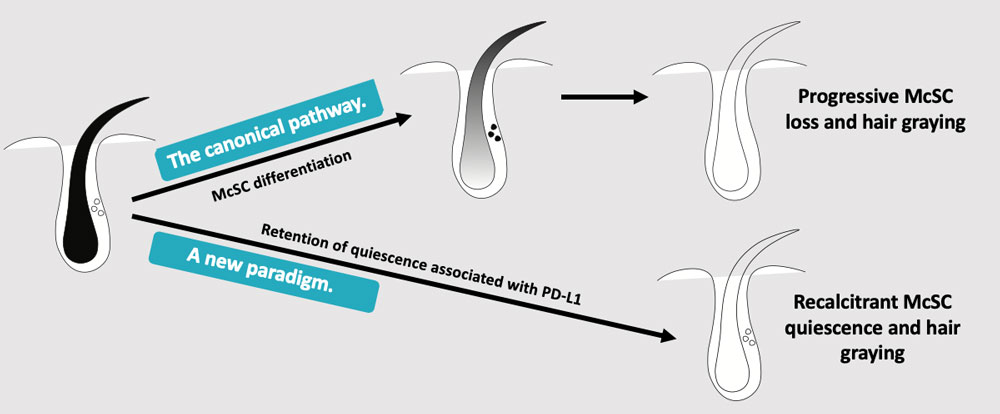 Image courtesy Melissa Harris
Image courtesy Melissa Harris
Wake up, little sleeper
The Harris lab looked at quiescent melanocyte stem cells and found that they expressed more of the PD-L1 protein than actively dividing cells. “And as you go farther into quiescence, cells are expressing more PD-L1, and they are harder to reactivate when Joseph gives them cues to say, ‘Hey, wake up!” Harris said.
The research has convinced Harris that a new paradigm is at play. The canonical pathway — progressive melanocyte stem cell loss and hair graying — making way for a new paradigm of recalcitrant melanocyte stem cell quiescence and hair graying. “This suggests we can find therapies to reactivate them,” Harris said. The compound she is investigating with the biotech startup could be a promising way forward.
“We have an opportunity with this company to find out what are the potential ways we can fix a broken system,” Harris said. “We’re always looking at what’s broken and rarely do we get to go in the other direction, towards tissue rejuvenation. So, this is exciting.”
Read more about the work Melissa Harris's fellow K99/R00 'kangaroo' awardees are doing at UAB:

Young investigator’s ‘kangaroo’ vaults her aging research onto a new level

‘I was tired of being the only’: words and deeds that can save science careers

