The Drug Discovery and Device Development Program represents a collaboration among the CCTS, the Heersink School of Medicine, the O'Neal Comprehensive Cancer Center, and the Alabama Drug Discovery Alliance (ADDA). It currently has several projects underway at different stages of development ranging from assay development and validation, high-throughput screening (HTS), lead identification and development, and a Phase I clinical trial.
The program also supports an annual Drug Discovery Research Award--projects are identified on a competitive basis in a two-stage application process overseen by ADDA. Funding typically involves at least two partners, including CCTS and Southern Research, a not-for-profit institution with an internationally recognized track record in drug discovery and development. Eligibility for the award has been for scientists affiliated with UAB or Southern Research. Efforts are underway to explore how CCTS partner institutions can work together with Southern Research to initiate similar research initiatives and high throughput screening campaigns for novel targets of interest.
Drug Discovery Research Award
The ADDA is no longer accepting pre-proposals for its Pilot Grants in Drug Discovery and Development. The 2-page pre-proposals were due Thursday, November 2, 2017, by 12:00 noon. All UAB and Southern Research investigators are eligible to apply, regardless of rank. Complete eligibility and pre-proposal instructions are available here.
Grants are for $50,000 per year for 2 years, with the second year of funding dependent on progress made in the first year. Funds can be utilized for various aspects of drug discovery and development for any human disease, including - but not limited to - assay development for high-throughput screening (HST), molecular modeling and medicinal chemistry efforts, or proof of concept studies in animals with newly discovered lead molecules. Only small molecule-directed efforts are eligible for funding at this time.
Upon funding, a multidisciplinary team is assembled, consisting of members with relevant expertise, including cell biology, pharmacology, medicinal chemistry, HTS assay development as well as clinical knowledge. This team, which meets quarterly, helps create a compound progression pathway and guide the project through the drug discovery and development pipeline.
In addition to scientific merit, applications are evaluated for intellectual property and commercialization aspects, in collaboration with the UAB Institute for Innovation and Entrepreneurship and Southern Research.
To hear about these and other funding opportunities, subscribe to our CCTS Digest.
The CCTS wants to help position investigators to apply for Department of Defense (DoD) Congressionally Directed Medical Research Program (CDMRP) announcements. View the 2015 targeted research areas and funding available.
How can the CCTS help? Last year we assembled a group of investigators who have either received DoD funding or reviewed DoD applications to discuss the application process (administrative issues, reviewer insights, areas of emphasis in review, etc.). View the handouts prepared for the meeting and helpful notes from attending. We have also set up a library of successful applications that we received permission to share (BlazerID required for access).
At any time, the CCTS can facilitate Project Panels to help with Letters of Intent or applications.
Remember - all extramural funding proposals must come to the OSP: 7 business days for the draft; 3 business days for the final. Questions? Contact
Funded CDMRP projects
- Search for funded research.
- View research highlights and videos.
- Read abstracts from "game-changing" funded projects.
Current Program Announcements
Preproposals are required and full proposals are by invitation only. Please see the instructions and program announcements. Links to both are HERE. Program Announcements are posted initially on Grants.gov - enter keyword as 12.420.
Sharpening Your Science
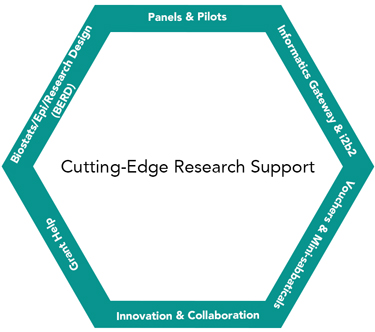 The CCTS Research Commons domain connects investigators to the expertise and technology they need, including to inter-institutional resources, consultations, and collaborators across our Partner Network. The goal? To sharpen science and accelerate the pace of discovery, thereby improving patient care and reducing the disparities and diseases that disproportionately affect our tri-state region (Alabama, Louisiana, and Mississippi).
The CCTS Research Commons domain connects investigators to the expertise and technology they need, including to inter-institutional resources, consultations, and collaborators across our Partner Network. The goal? To sharpen science and accelerate the pace of discovery, thereby improving patient care and reducing the disparities and diseases that disproportionately affect our tri-state region (Alabama, Louisiana, and Mississippi).
Whether you are a student or trainee, a full professor, or somewhere in between, we offer a wide array of expert research supports:
Informatics
CCTS Informatics comprises bioinformatics and clinical informatics experts who collaborate and consult with investigators across the translational spectrum. From study design to data access and analysis, we offer innovative tools and techniques that will take your research to a new level. Learn more
Biostatistics, Epidemiology, and Research Design (BERD)
Comprising a multidisciplinary team of expert biostatisticians, epidemiologists, and methodologists, our BERD unit supports rigorous methodology and scientific reproducibility in clinical and translational research. Learn more
Panels
Wherever you are in the research process, there is a CCTS Panel that will help you strengthen the aims and rigor of your grant application or project. Investigators who receive a CCTS panel are three times more likely to receive a fundable score than the NIH baseline funding rate. Learn more
Funding
Our CCTS Interdisciplinary Network Pilot Program helps accelerate projects with potential to move the needle on mission-relevant health outcomes. Our vouchers offer modest direct research support to offset expenses for clinical and translational research services, and our mini-sabbaticals provide "immersion" experiences for investigators in need of new experiential knowledge, skills, or collaborations. Learn more
Grant Help
From idea to implementation, we help you develop proposals that get funded and result in impactful projects. See our Grant Library for examples of successful applications to federal agencies, including NIH, NSF, and DoD, and private funding organizations. Learn more
Research Implementation
We support clinical and translational investigation with a variety of high-quality clinical services, including our Clinical Research Support Program. Learn more
Commercialization
Our I-Corps@NCATS Regional Short Course and I-Corps Bootcamp help investigators consider the value proposition of their discoveries and get out of the lab to meet potential customers. We also offer a special Innovation Panel (iPanel) that introduces scientists to the intellectual property pathway. Learn more
Trainings
We offer an extensive menu of learning opportunities that promote the continuous development of skills and knowledge for learners at all career stages in support of a vibrant, innovative, multidisciplinary research workforce to improve human health and health care. Learn more
Don't see what you need? Contact us at
From Ideation to Implementation and Commercialization
- Do you need help calculating sample size and power for your study design?
- Do you have a research idea but want to check cohort feasibility?
- Do you want to increase the rigor of your research?
- Do you seek a first-level peer review of your grant?
- Are you wondering how to create a well-crafted budget or data management plan for your grant or protocol?
- Do you need proposal development support?
- Do you want to explore clinical data for potential participants that match your eligibility criteria?
- Have you received a notice of grant award (NOGA) and need guidance on how to implement your study?
- Have you made a discovery with commercial potential, but aren't sure where to begin on the IP (intellectual property) pathway?
Through the CCTS Research Commons, we can connect you to a breadth and depth of expertise that will help you improve your grant scores, conduct a successful study, and increase the impact of your science. Contact us at