
Emmy Bell, MD, MSPH
Emmy Bell, MD, MSPH
Professor,
Division of Nephrology
Digital Spatial Profiling of Hypertensive Arterionephrosclerosis


Jennifer Caldwell, PhD, MPH
Jennifer Caldwell, PhD, MPH
Assistant Professor,
Public Health
Assessing feasibility of including community members in User Experience (UX) design for Genetic testing digital platform


Denise Danos, PhD
Denise Danos, PhD
Assistant Professor, Interdisciplinary Oncology
Building a software prototype for practice-based clinical trial matching to improve enrollment


Christy Foster, MD
Christy Foster, MD
Assistant Prof,
Pediatric Endocrinology
Evaluation of a recruitment method for an underrepresented adolescent population for a clinical study for genetic studies


Sadis Matalon, MS, PhD, ScD
Sadis Matalon, MS, PhD, ScD
Professor,
Anesthesiology and Perioperative Medicine
Optimization of Recombinant Hemopexin for the Mitigation of Chemical Lung Injury


Peter Morris, MD
Peter Morris, MD
Professor
Pulmonary, Allergy, and Critical Care Medicine
Addressing Inpatient Trial Design Translational Barriers through Refining Post-ICU Recovery Trajectories to Inform Hospital Re-Admissions


Seung-Yup (Joshua) Lee, PhD
Seung-Yup (Joshua) Lee, PhD
Assistant Professor
Department of Health Services Administration
Empirically-Grounded Referral Optimization for Effective Diabetes Care Coordination and Glycemic Management

Health outcomes are often affected by more than biology. They can be influenced by conditions in which people are born, grow, live and work. Understanding how biologic, clinical, economic, educational, structural and social factors relate is critical to elevate health equity for all. The CCTS connects investigators and teams to the expertise and tools to conduct rigorous research involving social determinants of health.
UAB's Geographic Information System (GIS) is a tool that assists in plotting and analyzing geographical data and is useful in decision-making processes across fields like urban planning, environmental management, and business logistics.
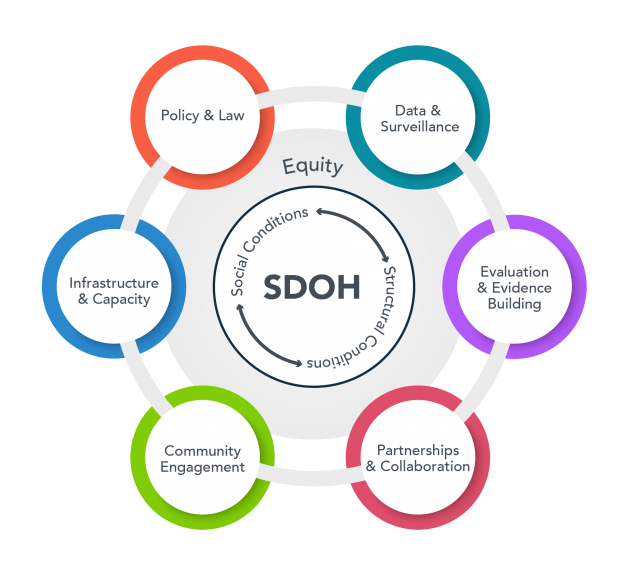
The Social Determinants of Health (SDH) Core empowers UAB investigators to evaluate how social and environmental factors influence the onset, development, management, and outcomes of diseases. It also promotes the development of interventions that mitigate these factors. By combining integrated data, methodologies, and expertise from various fields such as social science, spatial and environmental science, clinical and translational science, genomics, informatics, and epidemiology, we pave the way for pioneering investigations into the genome-sociome-exposome pathways linked to health and disease.
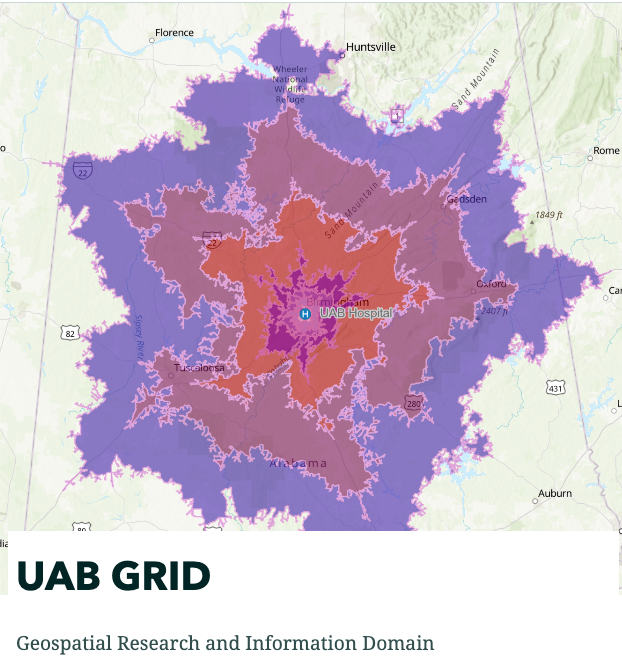
The CCTS’s GRID (Geospatial Research and Information Domain) provides access to tools and resources to utilize Geographic Information System (GIS) software that allows investigators to analyzes and map all types of data. Used across various industries, GIS helps in understanding patterns, relationships, and geographical contexts, thereby enhancing communication, decision-making, and efficiency. GIS offers a more dynamic understanding of the world, aiding in informed decision-making by translating complex data into a more comprehensible visual format.
-
How to Log in to DataLENS
1. Go to DataLENS at https://datalens.hs.uab.edu/projectexplorer
2. Log in using @uabmc.edu account if possible. If you do not have @uabmc.edu account, use your @uab.edu account. Please emailThis email address is being protected from spambots. You need JavaScript enabled to view it. if you encounter any issue logging in. -
Requesting Datasets
-
After logging in, go to “My Data Request” tab to create new request by clicking “+NEW DATA REQUEST” button.
-
Fill out the data request form.
-
Project Name: any data request needs to be tied to a project. If you have not create any project, you can create new project by clicking the [+] button.
-
Select Cohorts: Please select your query that you ran in i2b2 that defines your cohort of the request. This is optional.
-
Select Protocols: Please select your IRB number from the dropdown list if you are requesting PHI. This is optional.
-
Title: Title of your data request
-
Description: detailed description of your data request. Below key information are usually needed in a data request:
i. Define your study population. (e.g. African American women over 50 years old with type 2 diabetes, white males diagnosed with myocardial infection in past 60 days.)
ii. What data do you want on your study population? Click here for a list of searchable data variables.
iii. What time frame should your data cover? (e.g. past 3 months, 2011-2016) -
Status: This is a read only field and the status of your request will updated when analysts begin working on your request.
-
Priority: This is a read only field. Normal priority by default. If your request is urgent, please indicate it in the Description along with reason and justification.
-
Assignee: This is a read only field. You should see analyst name when it is worked on.
-
Frequency: How often would you like this data to be refreshed and delivered? It is a one-time request by default.
-
Requester Name: Type in <Last Name>, <First Name> to search for matches in database. By default, it will be the person who submitted the request.
-
Attach Files: Please attach IRB approval letter and Human Subjects Protocol (or EForm of e-Portfolio) if you are requesting PHI. This will speed up our validating process. For large files, please wait until you see an “upload successful” message appears on the top right corner of the screen.
-
Documents: You should be able to see any documents you have uploaded previously.
-
-
Click “Submit” button to complete request creation process. If you have attached files, please make sure you see the “upload successful” message before you click “Submit” button. This will prevent loss of the attachments.\
-
Our analysts will process your request in the order of it is received. You may check the Status in your request. Once completed, the dataset files will be delivered in the “Clinical Datasets” tab.
-
-
Troubleshooting
Please email
This email address is being protected from spambots. You need JavaScript enabled to view it. if you encounter any issue logging in and use this registration form for data requests should you need it.
Trouble accessing? Contact
Translational Science
Translation is the process of ‘turning science into health.’1 Translational science is systematically addressing a major barrier(s) that prohibits translating research from one stage to the next. The goal is to address common causes of inefficiency and failure in research (i.e. translational barriers) to elevate the efficiency and effectiveness of getting more treatments to more people. Some examples of translational barriers include lack of patient/community engagement in the development and implementation of health interventions, ineffective participant recruitment and retention, lack of data fidelity, interoperability and/or transparency, failure in clinical trial design, and incorrect predictions of drug toxicity and efficacy.
Translational Science in Pilot Projects
Translational Barrier: Novel Clinical Trial Design
In several clinical settings, physicians lack evidenced-based methods to predict health outcomes, especially at the point of care, which represents a major translational barrier towards examining the efficacy of health interventions (i.e. clinical trial design). Dr. Peter Morris, MD, Professor in UAB’s Department of Pulmonary, Allergy and Critical Care Medicine, is addressing this barrier by identifying clinical predictors of poor health outcomes. Using sepsis survivors as the initial use-case, Dr. Morris and team will conduct an observational, prospective study to assess if physical function recovery trajectory and rehospitalization events can inform the development of post-intensive care unit recovery subgroups. Establishment of these subgroups could inform a novel approach to clinical trial design within and beyond the context of sepsis.
 Translational Barrier: Fidelity of remote data collection.
Translational Barrier: Fidelity of remote data collection.
 Translational Barrier: Patient Recruitment
Translational Barrier: Patient Recruitment
Clinical trial failure is frequently attributed to lack of patient recruitment, particularly amongst minority pediatric populations. Dr. Christy Foster, MD, Assistant Professor at in Pediatric Endocrinology at UAB, aims to address this translational barrier by examining the feasibility of a novel recruitment method – adolescent peer recruitment. Specifically, Dr. Foster and team will evaluate the feasibility of peer recruiters improving clinical study engagement of adolescents with similar age, background, and experiences. The results of this study will inform the development of a novel recruitment method, which are desperately needed as part of clinicians providing evidence-based health interventions to the populations most disparately impacted.
 Translational Barrier: Patient Recruitment
Translational Barrier: Patient Recruitment
With modest pilot funds, investigators can systematically address barriers in their research plans, thereby developing more predictive and successful health interventions. Is there a barrier inhibiting your research from moving one stage to the next?
For additional guidance on translational science, check out the publications listed below, read about the CCTS Pilot Program and listen to this webinar, or contact your CCTS (

Annabelle Fonseca, MD, MHS
Asst. Professor, Dept. of Surgery

Byron Lai, PhD, MS
Asst. Professor, Dept. of Pediatrics, Div. of Pediatric Rehabilitation Medicine

Chad Rose, PhD
Asst. Professor, Dept. of Mechanical Engineering; Director, Wearable and Bio-Robotics Lab (WeBR Lab)

Henry Zelada Castro, MD
Asst. Professor, Dept. of Medicine, Div. of Endicrinology, Diabetes and Metabolism

Jaimie Roper, PhD
Asst. Professor, Dept. of Kinesiology; Director, Locomotor and Movement Control Lab 

Mohamed Shaban, PhD, MS, MS
Asst. Professor, Dept. of Electrical and Computer Engineering; Director, Biomedical Artificial Intelligence Group