 |
Gorgas Case 2014-08 |
 |
| Diagnosis: Sporotrichoid leishmaniasis due to L. braziliensis. |
|
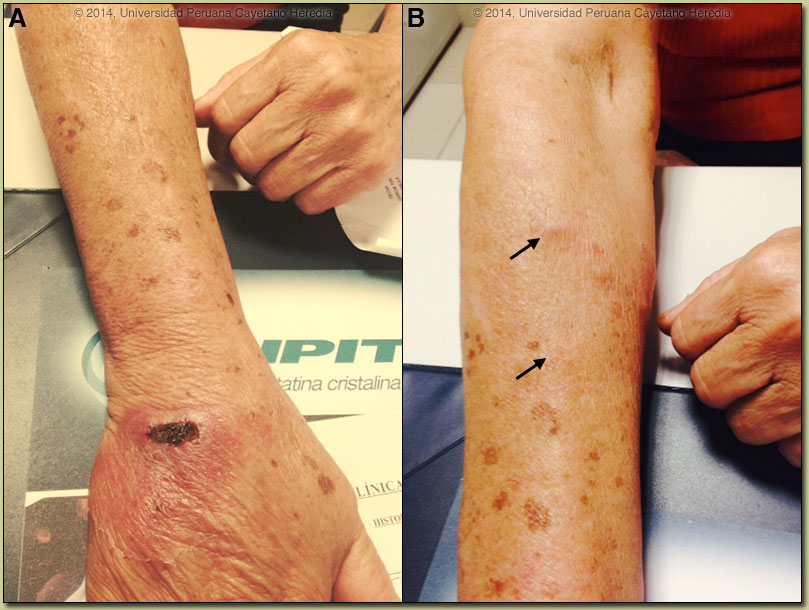
The major differential given the onset of the lesions on the hand and the lymphatic distribution would include leishmaniasis [see a classic skin ulcer in Gorgas Case 2009-01], sporotrichosis [as in Gorgas Case 2008-01], atypical mycobacteria, and nocardiosis. In Perú, leishmaniasis would be by far the most common, and ulcerative lesions are typically painless. This patient’s occupational exposures in the aquaculture industry made atypical mycobacteria the initial clinical diagnosis when she arrived. In South America it is important to distinguish Leishmania species that cause only cutaneous disease from the mucocutaneous species. Both cause initial skin ulcers but with mucocutaneous species, from months to years after treatment or healing of the skin ulcers, severe destructive recurrence may occur in the mucosal surfaces of the naso- and oropharynx [see Gorgas Case 2013-02]. In the Perúvian jungle where this patient lives, leishmaniasis is essentially exclusively due to Leishmania braziliensis, a species causing mucocutaneous disease. In this part of the world the vector is the Lutzomyia sand fly. In Perú L. peruviana (almost exclusively cutaneous disease) occurs in the high Andes; L braziliensis occurs predominantly in the jungle and in the Amazon. Distinguishing L. braziliensis from L. peruviana (and from less frequently occurring species) had for many years involved laborious culture techniques followed by electrophoretic isoenzyme analysis. Investigators at our Institute have now published PCR assays using both tissue as well as less invasive specimens [PLoS One. 2011;6(10):e26395; Clin Infect Dis. 2010 Jan 1;50(1):e1-6] for distinguishing the two species from cutaneous and mucosal lesions. Our patient was treated with a standard course of pentavalent antimony (20 mg/kg/d) per 20 days. The lesion was almost completely healed by the end of therapy and one-month after completion of treatment there was complete resolution of all lesions [Image D]. Specific identification of the species that causes the initial cutaneous infection has been considered important as systemic treatment has been thought necessary to prevent mucocutaneous leishmaniasis due to L. braziliensis. Recently, the concept of whether the risk of developing mucosal leishmaniasis (ML) warrants systemic treatment in all patients with New World cutaneous leishmaniasis (CL) has been called into question [Int Health. 2012 Sep;4(3):153-63]. Increasing experiences with local therapy, and the lack of proof of significant risk of mucosal dissemination with often-toxic systemic therapy, have fueled this debate. Local treatment might be considered for travelers with cutaneous L. braziliensis, if they have mild disease, single small lesions (<5 cm), are immunocompetent and begin to heal rapidly with only local or no therapy. |
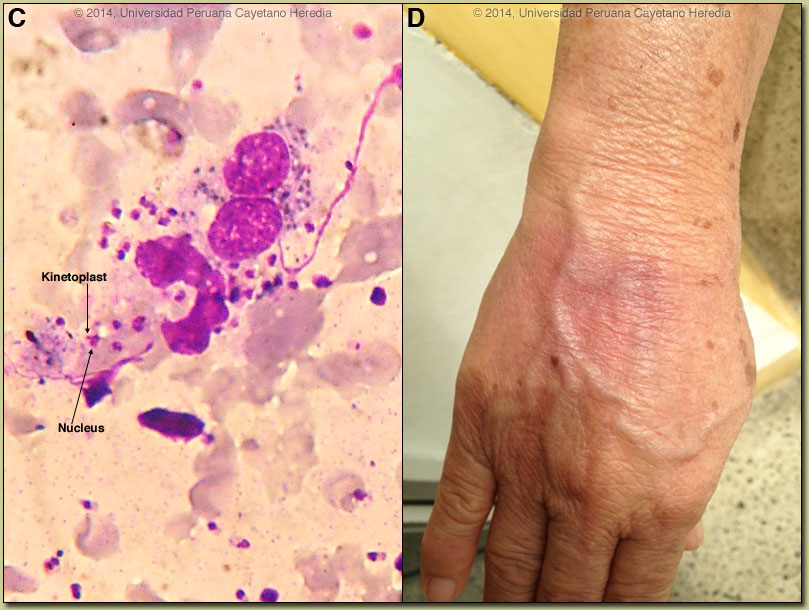 Discussion: Giemsa stain of a needle aspirate from the border of the lesion disclosed many intracellular amastigotes of Leishmania [Image C]. The arrows denote the characteristic and diagnostic kinetoplast (specialized DNA) that is very small and completely distinct from the nucleus. A culture was positive for L. braziliensis. Routine cultures and stains were negative as were AFB and fungal cultures.
Discussion: Giemsa stain of a needle aspirate from the border of the lesion disclosed many intracellular amastigotes of Leishmania [Image C]. The arrows denote the characteristic and diagnostic kinetoplast (specialized DNA) that is very small and completely distinct from the nucleus. A culture was positive for L. braziliensis. Routine cultures and stains were negative as were AFB and fungal cultures.