Hoover resident Jack Brymer was driving home with his wife, Shirley, one night recently when it became clear to him his health problem was getting worse at a rapid pace.
 Jack Brymer“I had been having dizzy spells, and it would feel like I was going to faint, and it was a problem that just kept getting worse,” said the 78-year-old Brymer. “That night, it got so bad that I pulled over. I couldn’t drive because I felt like I was going to faint.”
Jack Brymer“I had been having dizzy spells, and it would feel like I was going to faint, and it was a problem that just kept getting worse,” said the 78-year-old Brymer. “That night, it got so bad that I pulled over. I couldn’t drive because I felt like I was going to faint.”
University of Alabama at Birmingham electrophysiologists examined Brymer and determined the bottom chambers of his heart would periodically pause for up to five seconds, a dangerous condition which could ultimately lead to a total heart stoppage, which would require a pacemaker. UAB physicians ultimately determined that Brymer was a candidate for the new Nanostim™ leadless pacemaker, the world’s first retrievable, nonsurgical pacing technology. The first two pacemakers of this kind in Alabama were implanted into Brymer and another patient at UAB Hospital earlier this month.
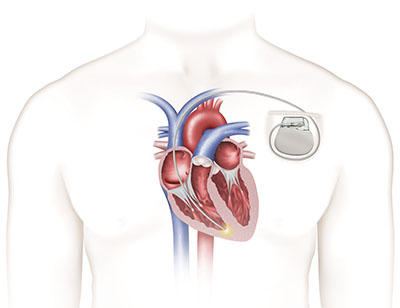 Traditional pacemaker
Traditional pacemaker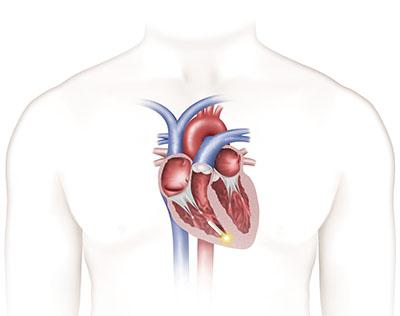 Nanostim leadless pacemaker“It’s not very often that a new therapy comes along that has as much potential as this new, leadless pacemaker does,” said Vance Plumb, M.D., professor of medicine in the Division of Cardiovascular Disease in the School of Medicine. “Historically, the pacing leads have been the weak link causing failure, which this device eliminates. It’s a big step forward in patient treatment and a milestone for cardiac rhythm treatment in Alabama.”
Nanostim leadless pacemaker“It’s not very often that a new therapy comes along that has as much potential as this new, leadless pacemaker does,” said Vance Plumb, M.D., professor of medicine in the Division of Cardiovascular Disease in the School of Medicine. “Historically, the pacing leads have been the weak link causing failure, which this device eliminates. It’s a big step forward in patient treatment and a milestone for cardiac rhythm treatment in Alabama.”
Developed for patients with bradycardia — a heart rate that is too slow — the Nanostim device is designed to be placed directly on the patient’s heart without the visible lump, scar and insulated wires, or leads, required for conventional pacemakers.
Brymer’s implant took place as part of the LEADLESS II pivotal trial, a prospective, nonrandomized, multicenter, international clinical study designed to evaluate the safety and effectiveness of the St. Jude Medical device in patients indicated for the device in the United States. The study is expected to enroll approximately 670 patients at 50 centers.
“Most of the patients who will be candidates for the leadless pacemaker will have atrial fibrillation and pauses in their heartbeat,” said Harish Doppalapudi, M.D., assistant professor of medicine and director of the Clinical Cardiac Electrophysiology Training Program, who implanted the first Nanostim device in Alabama. “These patients only need pacing in the bottom chamber of the heart, which is what the leadless pacemaker provides. It’s a groundbreaking technology that should improve patient care and minimize complications.”
Complications at implant should be minimized because implanting the device is a less-invasive approach compared to traditional pacemaker procedures that require more extensive surgery. Leadless pacemaker technology is made up of computer chips and a small, but long-lived battery in a sealed case. The device is implanted through a vein that passes fairly close to the outer surface of the upper thighs. Because the implant procedure does not require surgery like a traditional procedure, it is considered a less-invasive approach for patients who need pacemaker technology.
“Other patients who should benefit from this device include those with difficult or limited vascular access for conventional pacing lead placement, patients who are prone to infections, including those with transplants, kidney failure or on dialysis, and patients who can be anticipated to rarely need upper chamber pacing,” Plumb said.
 “Because there is no device under the skin, there is no visible scar and no risk of erosion, a safety feature that is not achievable with traditional pacemakers,” Doppalapudi added. “The quality of life for patients who receive the leadless pacemaker should be better than that of patients who get conventional pacemakers. There is no pain in the shoulder, no scar or lump, and no activity restrictions of the arm and shoulder. In fact, most will continue to live active, uninhibited lifestyles.”
“Because there is no device under the skin, there is no visible scar and no risk of erosion, a safety feature that is not achievable with traditional pacemakers,” Doppalapudi added. “The quality of life for patients who receive the leadless pacemaker should be better than that of patients who get conventional pacemakers. There is no pain in the shoulder, no scar or lump, and no activity restrictions of the arm and shoulder. In fact, most will continue to live active, uninhibited lifestyles.”
The Nanostim leadless device is fully retrievable so that it can be readily repositioned during the implant procedure and later retrieved if necessary. Although the pacemaker is less than 10 percent of the size of a conventional pacemaker, the projected battery life is comparable and it is the least invasive pacing technology available today.
The device is supported by the St. Jude Medical Merlin™ Programmer, which is also used to interrogate and program the company’s other pacemakers and implantable cardioverter defibrillators, or ICDs. More than 4 million people worldwide have an implanted pacemaker or other cardiac rhythm management device, and an additional 700,000 patients receive the devices each year.
As for Brymer, he is happy to see a positive change in his health.
“I feel fine,” he said. “It’s been more than a week since the pacemaker was implanted, and I haven’t had any problems at all. I haven’t had any dizzy spells, and the procedure was quick, too. I went in at 7 a.m. and was home by 7 p.m. I went for a long walk a few days later with my wife. I told her for the first time in a long time I felt good again.”