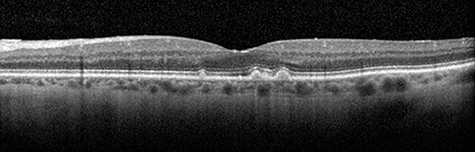
 Layers of the retina are shown by optical coherence tomography, as are drusen (bumps), the characteristic lesions of age-related macular degeneration (AMD)Researchers from the University of Alabama at Birmingham Department of Ophthalmology and Visual Sciences, along with collaborators from the University of Iowa, have discovered a genetic biomarker that is associated with age-related macular degeneration and delayed rod-mediated dark adaptation — the first visual function for incident AMD in older adults with normal macular health and early AMD.
Layers of the retina are shown by optical coherence tomography, as are drusen (bumps), the characteristic lesions of age-related macular degeneration (AMD)Researchers from the University of Alabama at Birmingham Department of Ophthalmology and Visual Sciences, along with collaborators from the University of Iowa, have discovered a genetic biomarker that is associated with age-related macular degeneration and delayed rod-mediated dark adaptation — the first visual function for incident AMD in older adults with normal macular health and early AMD.
According to the Centers for Disease Control and Prevention, AMD is a major cause of blindness worldwide and is the leading cause of vision loss and blindness for Americans age 65 years and older.
Professors Cynthia Owsley, Ph.D., and Christine A. Curcio, Ph.D., say there are no current proven strategies for preventing AMD or stopping its progression early in the disease when sight could be saved. Two of the strongest genetic associations for age-related macular degeneration are common polymorphisms — variants in DNA sequence — at chromosome 1 (CFH) and chromosome 10 (ARMS2).
“We have previously shown that delayed rod-mediated dark adaptation is the first functional risk factor for early AMD,” said Owsley, the Nathan E. Miles Chair of Ophthalmology. “Delayed dark adaptation means it takes these individuals much longer to adapt to a dark environment — for example, after entering a darkened movie theater — than other individuals. This was important, because vision in bright light was known to be relatively preserved late into the disease. Night vision is affected much earlier. ”
In other words, older adults with delayed dark adaptation have a heightened risk for developing AMD within the next few years.
In the recently published study, Owsley and Curcio, with collaborators Robert F. Mullins and Edwin M. Stone of the University of Iowa, established that older adults with delayed dark adaptation are also more likely to have these high-risk genetic polymorphisms at chromosome 1 and chromosome 10.
“This finding was the first genotype-functional phenotype association found in AMD research,” Owsley said. “What we find particularly exciting is that the ARMS2 genotype-phenotype association emerges even at pre-clinical stages of AMD — that is, in older adults who do not yet have AMD. Being able to assess risk at such an early stage could lead to new preventive measures.”
Owsley says the ARMS2 gene is poorly understood from a biological standpoint and is also challenging to study because it is not expressed in adults.
“However, our study suggests that making ARMS2 a research priority will lead to new ways of tackling AMD and developing treatments to prevent this disabling disease,” she said.
Funding for this research was provided by National Institutes of Health grants, the Dorsett Davis Discovery Fund, the National Center for Advanced Translational Sciences of NIH, the Alfreda J. Schueler Trust, the EyeSight Foundation of Alabama, Research to Prevent Blindness and the Macula Foundation.