-
UAB Comprehensive Diabetes Center starting denosumab trial in adults with established Type 1 diabetesThe Denosumab for Type 1 Diabetes phase 1/2 clinical trial, is a collaborative study with the Arthur Riggs Diabetes and Metabolism Research Institute and the Center for Diabetes and Metabolic Diseases at Indiana University.posted a while back 1256 viewsMost teens with autism do not drive. This researcher is testing a way to change that.Emma Sartin, Ph.D., MPH, assistant professor in the Department of Health Policy and Organization at the School of Public Health, is leading a Department of Defense-funded project that is developing a virtual assessment to help autistic individuals and their parents decide whether they are ready to drive.posted a while back 2025 viewsMeet the leader of UAB’s Climate and Health InitiativeZhen Cong, Ph.D., professor in the Department of Environmental Health Sciences in the School of Public Health, shares her work on disaster preparedness of older adults and why she wants all Blazers to take part in the initiative’s work.posted a while back 1616 viewsThis neuroscientist is starting seizures in order to stop themRachel June Smith, Ph.D., a key recruit in UAB’s Neuroengineering and Brain-Computer Interface Initiative, can predict the frequency of stimulation that will push a brain into the chaos of a seizure — potentially saving patients with intractable seizures time, frustration and money.posted a while back 3456 views4 cutting-edge machines powering UAB discoveriesWith research awards breaking all-time records, we toured labs where high-tech tools are driving science forward.posted a while back 4085 viewsMeet the UAB doctors protecting Alabama from killer fungi
Alabama is a hotbed for fungal diseases — which is why experts in treating and tracking problematic fungi gravitate to UAB. This is great news for Alabamians as killer fungi become a worldwide threat.
posted a while back 8247 viewsFive lessons from the first five years of the All of Us Research ProgramHow the ambitious NIH initiative is turning precision medicine dreams into reality for hundreds of thousands of Americans left behind by previous studies — and where it is going next.posted a while back 6652 viewsAmid global ChatGPT obsession, UAB conference examines AI’s role in advancing health care and researchExperts at ATTIS 2023 shared reports from the front lines, including how they are using ChatGPT in their labs, the need for regulation and why this is a “tremendous time” for health care.posted a while back 4458 views“Dr. Impossible” aims to bring brain tech to the peopleTake a trip into the Alabama BRAIN Lab in UAB’s Spain Rehabilitation Center, where a team led by neuroengineer Jamie Tyler, Ph.D., is working with patient groups to test promising neuromodulation treatments for chronic pain, insomnia and more.posted a while back 8538 viewsWhy do super-agers skew female? These researchers are drilling into the hottest hypotheses.In a search that encompasses geckos, bats, sex-switching fish and more, the NSF-funded IISAGE team is seeking data to explain lopsided lifespans. A key question: How much wiggle room is there in aging?posted a while back 5053 viewsThis med student is on a mission to make tattoo inks safeA chemical mystery drew Matthew Kiszla into tattoo research: Why are red inks most likely to cause rashes and other reactions? Now he is working to analyze commercial inks and looking for collaborators both scientific and artistic.
posted a while back 14331 viewsInnovation Institute hosts country’s first metahealth symposium, Feb. 28The event will explore a future of health care that “is going to be anchored in the metaverse” and be the first such symposium to be held both live and in the metaverse itself, according to Rubin Pillay, M.D., Ph.D., executive director of the Marnix E. Heersink Institute for Biomedical Innovation.posted a while back 3874 viewsFour high-tech health devices being tested at UAB nowTake a look at new technologies being studied at UAB for treatment of depression, sleep apnea, traumatic brain injury and tic disorders.
posted a while back 5813 views3 UAB chemists break down their formulas for fighting cancerNew treatments emerging from the labs in the Department of Chemistry rely on split-second timing, tiny cargo bubbles and supercomputer-powered predictions.posted a while back 5369 viewsResearchers hack adaptive cruise control, then show how to make it saferDriver assistance tech that comes standard on new vehicles can be tricked into causing accidents — but there is a way to alert humans in time. A UAB grad student and his mentor will share their findings this month at a global conference.
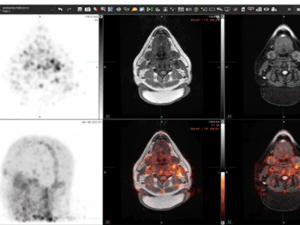
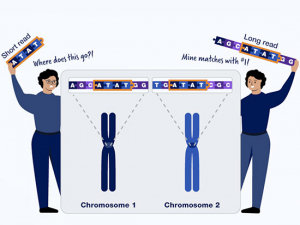
posted a while back 7529 viewsFirst-of-its-kind scan tracks immune invaders to explore puzzling brain diseasesPatients with multiple sclerosis, fibromyalgia and chronic fatigue syndrome will soon be able to enroll in the clinical trial of a PET agent that can capture evidence of brain infiltration by white blood cells and could eventually guide treatment.posted a while back 5883 viewsSalt boosts blood pressure for some people. UAB study asks: Who?By alternating high-salt and low-salt diets, a new clinical trial aims to find out how common salt sensitivity of blood pressure is in the general population. The researchers are also exploring whether the immune system plays a role.posted a while back 14621 viewsResearchers pioneering long-read sequencing studies explain why long reads matterNew technologies are filling in gaps in the human genome and opening major areas for discovery. Zechen Chong, Ph.D., and Robert Kimberly, M.D., explain the pros and cons and how they are using long reads at UAB.posted a while back 8560 viewsUAB study: Could this five-second obesity management strategy keep the pounds off?American adults tend to gain a pound or two per year. Researchers are testing a new approach to halt this creeping weight gain. They give participants a digital scale that graphs their weight over time and one job: step on it daily.posted a while back 13700 viewsThis long-running study proves that nice people finish firstMore than 100 different UAB researchers have been first authors on papers based on the REGARDS study thanks to its innovative design — and a uniquely “friendly and welcoming team.”posted a while back 8779 viewsMore research from UAB News
- Trials and tribulations of cell therapy for heart failure, an update on ongoing trials
- Study reveals the disruption of interaction between proteins CD2:CD58 is a potential new treatment option for people with hidradenitis suppurativa
- UAB Lung Health Center to target youth smoking and vaping with annual award from the ADPH