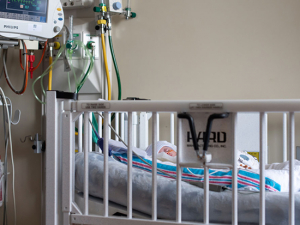
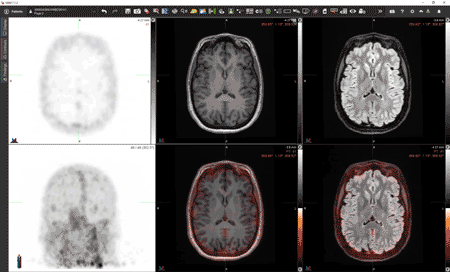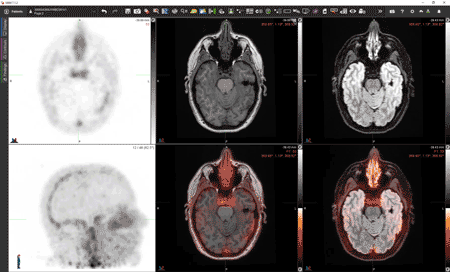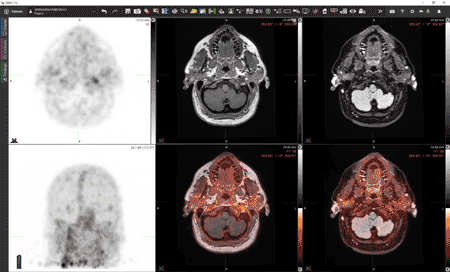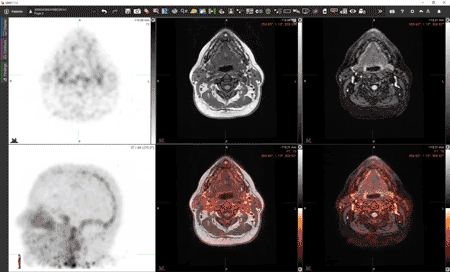
 Cross-sections of the brain reveal [Zr-89]oxine-labeled white blood cells in a new scan developed by UAB researchers. Video courtesy of Drs. Lapi, McConathy and Younger.After he had been at UAB for a few years, building a lab focused on brain inflammation and puzzling diseases, Jarred Younger, Ph.D., started asking a question.
Cross-sections of the brain reveal [Zr-89]oxine-labeled white blood cells in a new scan developed by UAB researchers. Video courtesy of Drs. Lapi, McConathy and Younger.After he had been at UAB for a few years, building a lab focused on brain inflammation and puzzling diseases, Jarred Younger, Ph.D., started asking a question.
“I said, ‘I need to track immune cells penetrating the brain — how could we do that?’” said Younger, professor in the Department of Psychology and director of the Neuro-inflammation, Pain and Fatigue Laboratory. At UAB, home to world-renowned specialists in neuroscience, immunology and brain imaging, that question prompted “a lot of different ideas,” Younger recalled. Many were persuasive in theory but, for various reasons, impractical for Younger’s purpose.
Then he connected with Suzanne Lapi, Ph.D., professor and director of the UAB Cyclotron Facility and vice chair of Research in the Heersink School of Medicine Department of Radiology, and Jonathan McConathy, M.D., Ph.D., division director of Molecular Imaging and Therapeutics, who specialize in exactly this sort of question. The positron emission tomography agent they developed, [Zr-89]oxine-labeled white blood cells, is a first-of-its-kind test now in human trials. It will soon enroll patients with multiple sclerosis, fibromyalgia and chronic fatigue syndrome. If all works as planned, [Zr-89]oxine could answer burning questions and help guide treatment in a wide range of diseases involving the immune system.
“What is actually wrong?”
 Jarred Younger, Ph.D., is interested in several diseases with unknown causes or high levels of diagnostic uncertainty, including multiple sclerosis, fibromyalgia, Gulf War syndrome and chronic fatigue syndrome.Younger is interested in several diseases with unknown causes or high levels of diagnostic uncertainty, including multiple sclerosis (the former) and fibromyalgia, Gulf War syndrome and chronic fatigue syndrome (the latter). “Patients get this diagnosis, but what is actually wrong?” Younger said. “With multiple sclerosis, what we think is happening is that immune cells called leukocytes, or white blood cells, are penetrating the blood-brain barrier, and they are not supposed to be there.” The brain has its own separate immune defenders, microglia and astrocytes. If leukocytes find a way to enter the brain, they could generate an ongoing inflammatory reaction (a possible cause of fibromyalgia, Gulf War syndrome and chronic fatigue syndrome) or attack brain cells directly (multiple sclerosis). “We suspect that immune infiltration happens in humans because it happens in animal models, but no one has shown it,” Younger said. The hope is that this scan will definitively show when immune cells infiltrate the brain.
Jarred Younger, Ph.D., is interested in several diseases with unknown causes or high levels of diagnostic uncertainty, including multiple sclerosis, fibromyalgia, Gulf War syndrome and chronic fatigue syndrome.Younger is interested in several diseases with unknown causes or high levels of diagnostic uncertainty, including multiple sclerosis (the former) and fibromyalgia, Gulf War syndrome and chronic fatigue syndrome (the latter). “Patients get this diagnosis, but what is actually wrong?” Younger said. “With multiple sclerosis, what we think is happening is that immune cells called leukocytes, or white blood cells, are penetrating the blood-brain barrier, and they are not supposed to be there.” The brain has its own separate immune defenders, microglia and astrocytes. If leukocytes find a way to enter the brain, they could generate an ongoing inflammatory reaction (a possible cause of fibromyalgia, Gulf War syndrome and chronic fatigue syndrome) or attack brain cells directly (multiple sclerosis). “We suspect that immune infiltration happens in humans because it happens in animal models, but no one has shown it,” Younger said. The hope is that this scan will definitively show when immune cells infiltrate the brain.
Typically, diagnosis and treatment of patients with multiple sclerosis relies on identifying characteristic lesions in the brain. “But after lesions have formed, it’s too late — the damage has already happened,” Younger said. “This new scan will hopefully show us where the attacks are occurring before the lesions develop.” That information could be used to guide treatment, including starting or increasing the dosage of therapies that dampen immune response or prescribing drugs that can strengthen the blood-brain barrier.
Something “we haven’t been able to see before”
While the process is technically very complex, the idea is conceptually straightforward. “We take a blood sample from a patient, extract the leukocytes, tag them with [Zr-89]oxine, reinject them into the patient, and then wait three to five days and see where they go,” Younger said. “If they appear in the brain, that will be highly informative. It will clearly show pathology that we haven’t been able to see before and would be proof that the blood-brain barrier is defective in the patient and that those cells are finding a way to push through.”
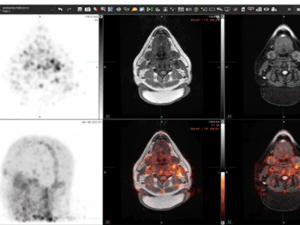
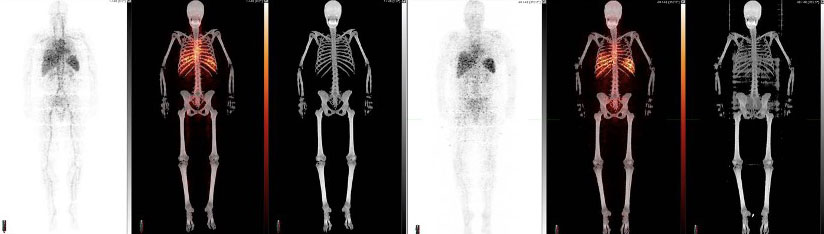 In the images above, a healthy participant is scanned at 5 minutes (three images at left) and 24 hours (three images at right) after receiving [Zr-89]oxine-labeled white blood cells. In each sequence, the first image is a PET scan, followed by a PET/CT and CT only. In the sequence at right, taken after 24 hours, the labeled white blood cells have homed to the bone marrow and spleen. Image courtesy of Drs. Lapi, McConathy and Younger.
In the images above, a healthy participant is scanned at 5 minutes (three images at left) and 24 hours (three images at right) after receiving [Zr-89]oxine-labeled white blood cells. In each sequence, the first image is a PET scan, followed by a PET/CT and CT only. In the sequence at right, taken after 24 hours, the labeled white blood cells have homed to the bone marrow and spleen. Image courtesy of Drs. Lapi, McConathy and Younger.
Lapi, Younger and McConathy have already performed [Zr-89]oxine scans on four healthy volunteers as part of their ongoing clinical trial. In the PET images from these scans, immune cells are seen throughout the body, circulating in the blood, and homing to bone marrow and spleen as expected (see image above). “But none of the [tagged] cells penetrated the brain,” Younger said. In other words, the healthy participants’ blood-brain barriers were functioning normally. Later this year, the team will test the scan in a patient with multiple sclerosis. “If it works, and the hypothesis is correct, we will see abnormal peripheral immune infiltration in the brain,” Younger said. “That will clearly be showing pathology that we haven’t been able to see before.”
Solving the problem
 Suzanne Lapi, Ph.D., points out features of one of the research scanners in UAB's Advanced Imaging Facility, just down the hall from its cyclotron, during a tour by Alabama Governor Kay Ivey.In PET imaging, scanners can be used to image the amount and location of positron-emitting particles, or radiotracers, in the body. Radiochemists like Lapi combine the tracers with biologically active molecules of interest. A common tracer, widely used with cancer patients, is F-18, fluorine 18, combined with glucose. The glucose accumulates preferentially in cancer cells, which are ravenous for nutrition because of their rapid growth.
Suzanne Lapi, Ph.D., points out features of one of the research scanners in UAB's Advanced Imaging Facility, just down the hall from its cyclotron, during a tour by Alabama Governor Kay Ivey.In PET imaging, scanners can be used to image the amount and location of positron-emitting particles, or radiotracers, in the body. Radiochemists like Lapi combine the tracers with biologically active molecules of interest. A common tracer, widely used with cancer patients, is F-18, fluorine 18, combined with glucose. The glucose accumulates preferentially in cancer cells, which are ravenous for nutrition because of their rapid growth.
Most medical centers have PET scanners. But UAB also has its own cyclotron, which produces both common tracers for clinical use and novel tracers for research. Lapi’s team develops new tracers and ways to connect them to molecules of interest. “We are really interested in making new isotopes to open the chemistry toolbox,” Lapi said. One of those isotopes is zirconium-89, or Zr-89, which is long-lived by PET imaging standards. For comparison, the half-life of F-18 is 110 minutes, while Zr-89 has a half-life of more than three days. Most PET tracers can image only processes that occur over minutes to hours, but Zr-89-labeled PET tracers can be used to image processes that occur over several days, including leukocyte movement throughout the body.
“I told the PET team what I was hoping to do, and Suzy said, ‘We work with zirconium 89, which will give us the amount of time we need, and oxine will get it into the cell,’” Younger said. “I posed the problem, and her team created the solution.”
The problem is “How do we radiolabel cells and figure out where in the body they go?” Lapi said. “People have looked at cell trafficking with various methods; but nobody has done it with this application, which is very sensitive, to look at cell trafficking into the brain.” The [Zr 89]oxine PET imaging agent could be applied to other research topics, Lapi points out, for instance, tracking stem cells or CAR-T cells, a form of immunotherapy used in cancer treatment.
Answering questions
In a paper published in 2021, Lapi’s team demonstrated their ability to produce [Zr-89]oxine in the quantities and following safety standards necessary for a human clinical trial, which had never been achieved before. They had to prove to the Food and Drug Administration, which regulates such investigational new drugs, that
- adding radio-emitting molecules to immune cells did not kill those cells,
- the cells went back to their previous behavior after being tagged with [Zr-89]oxine and
- the tags stayed in the cells rather than separating after injection into the participant.
“After a lot of work by a lot of people, including our lead chemist, Dr. Jennifer Bartels, and our lead radiopharmacist, Denise Jeffers, we have an approved clinical trial,” Lapi said.
If the initial studies are successful, a further step could be to tag only subsets of leukocytes in order to zoom in closer on causality. “We don’t exactly know which leukocytes could be causing the problem,” Younger said. “It could be T cells, B cells. Theoretically, this scan could work with any type of cell.”
Brain temperature scans
This is not the first new brain scan Younger has pioneered. A technique to measure brain temperature in patients using magnetic resonance spectroscopy, or MRS, is now widely used in Younger’s own research efforts and those of several other investigators at UAB.
“It’s a cool MRI trick,” Younger said. “Magnetic resonance spectroscopy measures the amount of different chemicals in the brain,” which appear on readouts as sharp peaks. Unlike other brain chemicals, the observed peaks of water shift as temperature rises. “That allows you to measure the temperature at any point in the brain and make a three-dimensional map,” Younger said. (See the scan and hear Younger describe it in this clip.)
Over the past four years, he has measured brain temperatures in more than 200 patients with rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome and traumatic brain injury, “and we just started testing long COVID,” he said. Other researchers at UAB are using the technique to gain new insight on epilepsy and post-traumatic stress disorder, among other conditions.
“We see the brain temperatures elevated one or two degrees” in these patients compared with healthy controls, Younger said. “The rest of the body looks fine; but when we scan the brain, we see this difference. That means they have inflammation in the brain. The question is ‘What is triggering it?’ One thought I had is that the blood-brain barrier has degraded and allowed immune cells to penetrate. They don’t know what to do in that environment, and they start driving this general inflammation. With [Zr-89]oxine, we will be able to put that hypothesis to the test.”