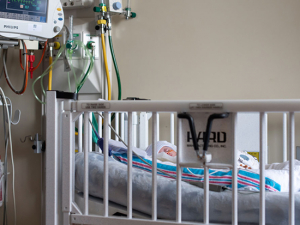
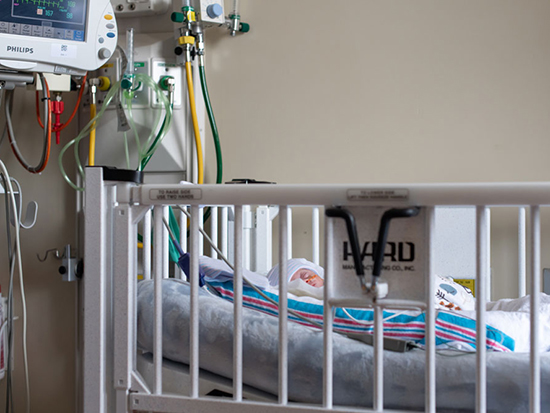
 A baby in UAB's Regional Newborn Intensive Care Unit in 2023, with a heart rate monitor partially visible at upper left. Premature babies often have difficulty breathing and slow heart rates known as bradycardia. A team of UAB clinician-researchers has created a device to predict and respond to bradycardia. With an investment from UAB's Blazer Bridge Fund, the team will now conduct a clinical trial and move forward with product development. Photo: Jennifer Alsabrook-Turner, UAB Marketing and CommunicationsPremature babies have difficulty controlling their breathing and have frequent episodes of apnea, when breathing temporarily stops. This causes a rapid slowing of the heartbeat, known as bradycardia, which can have severe, possibly fatal consequences. Because it reduces blood flow to major organs, bradycardia brings an increased risk of developmental issues and chronic lung disease.
A baby in UAB's Regional Newborn Intensive Care Unit in 2023, with a heart rate monitor partially visible at upper left. Premature babies often have difficulty breathing and slow heart rates known as bradycardia. A team of UAB clinician-researchers has created a device to predict and respond to bradycardia. With an investment from UAB's Blazer Bridge Fund, the team will now conduct a clinical trial and move forward with product development. Photo: Jennifer Alsabrook-Turner, UAB Marketing and CommunicationsPremature babies have difficulty controlling their breathing and have frequent episodes of apnea, when breathing temporarily stops. This causes a rapid slowing of the heartbeat, known as bradycardia, which can have severe, possibly fatal consequences. Because it reduces blood flow to major organs, bradycardia brings an increased risk of developmental issues and chronic lung disease.
The standard treatments for bradycardia in neonatal intensive care units, or NICUs, are methylxanthine drugs (caffeine) and continuous positive airway pressure machines, similar to the CPAP devices used for adults with sleep apnea. But these treatments have their own side effects. NICU staff also respond quickly to low-heartbeat alerts, gently touching the babies to stimulate them to resume breathing and increase their heart rates.
Predicting when breathing will stop
A group of UAB researchers has developed a better way. Studying thousands of hours of data from heartrate monitors, they developed algorithms to predict when a baby will go into bradycardia, up to 10 seconds before it happens. They built a device to respond to these warning signals, delivering a gentle stimulation (imagine the vibration on your cellphone when a text message comes in) through a padded bracelet. In a small study in UAB’s RNICU, they have already demonstrated that the algorithms are working well. The next step is a clinical trial to gather the data needed for a federal small-business grant and to demonstrate to venture capital firms that the approach works at scale.
The device clearly has the potential to meet an important need. Each year, more than a quarter-million preterm infants in the United States have a diagnosis of apnea of prematurity, and there are many-fold more around the world. It could be useful out of the hospital, as well. Many infants continue to have episodes of apnea after they go home; this is associated with an increased risk of sudden infant death syndrome, or SIDS.
Starting a critical clinical trial with Blazer Bridge Fund support
But clinical trials themselves are expensive, and a trial would require multiple devices. That is where the Blazer Bridge Fund comes in. The fund, created by UAB’s Bill L. Harbert Institute for Innovation and Entrepreneurship, just announced its second cohort of recipients. Each of the six projects selected for the cohort received $50,000 to support commercial development of a promising idea.
“The support of the Blazer Bridge Fund will be of great assistance in preparing our device for external funding and/or licensing opportunities,” said Colm Travers, M.D., one of the inventors of the Application to Predict and Prevent NEonatal Apnea (APPNEA), along with Namasivayam Ambalavanan, M.D., Arie Nakhmani, Ph.D., and Waldemar A. Carlo, M.D.
An experienced team
Travers, an associate professor in the Department of Pediatrics’ Division of Neonatology, has a strong track record of research in the mechanisms that control breathing and respiratory stability in infants. In 2022, he was first author on a paper in the journal Pediatrics detailing UAB’s Golden Week Program, a comprehensive, evidence-based quality initiative to reduce death and severe brain bleeding in extremely preterm infants that showed significant reductions in both measures. Ambalavanan, a professor and director of the Division of Neonatal Research in the Department of Pediatrics, directs single-center and multi-center clinical and translational studies in neonatal intensive care, focused primarily on control of breathing and lung development. Nakhmani is an associate professor in the Department of Electrical and Computer Engineering in the School of Engineering whose research focus is on developing computational tools for signal and image analysis using advanced mathematical models and machine learning. Carlo, professor and co-director of the Division of Neonatology at UAB, has extensive experience in collaborative single-center and large-scale clinical neonatal research, as well as designing and developing neonatal respiratory devices.
Support from the Blazer Bridge Fund will allow the APPNEA team to develop and design a revised prototype, complete the proposed clinical trials to optimize device efficacy, and develop a commercialization plan so that the team can improve outcomes for preterm infants in the United States and beyond, Travers says.
“APPNEA is a perfect example of a good cross-disciplinary team working on a translational solution to a very real problem,” said Wink Crittenden, Ph.D., licensing associate with the Harbert Institute for Innovation and Entrepreneurship. “Hopefully it’s going to change the standard of care for early and preterm babies.”