Reporter Staff
| This email address is being protected from spambots. You need JavaScript enabled to view it.Ladies We Need You!
Are you a cisgender woman over 18 who's been sexually active with a cisgender man in the past year?
If you haven't received a positive HIV test and haven't been on long-acting PrEP, you could be eligible to participate in our study.
Important: You CANNOT get HIV from participating in this clinical trial.Interested? Sign up today with the Alabama Vaccine Research Clinic at UAB to contribute to this vital research and get compensated for your participation. Call 205-934-7492 today.
ADAPT: A Clinical Trial of Adaptive Treatment for Early Smoking Cessation Relapse
https://redcap.musc.edu/surveys/?s=8JWFT9XFY3FYR7Y3
Day or Night? When is the best time to take your blood pressure medicine
Board codifies institutional neutrality
8 tips to enhance your quality of life as you age
3 chosen as UAB VIPs for third-quarter 2024
UAB employees who want to ensure their financial security in their golden years can take advantage of services provided by the UAB Benefits Office and UAB’s retirement providers, including retirement counseling and investment support for mandatory and voluntary retirement plans. If you are contacted by other vendors claiming to be authorized, do not give out your personal information.
Online submission for Equipment Disposition Forms starts Aug. 30
Participate in Type 2 Diabetes Research
About the Clinical Trial:
The purpose of this NIH funded trial is to examine the effects of three different diabetes treatments to determine if they improve night-time blood sugars.
This Trial Involves:
- A screening visit that includes a blood draw, urine test and physical exam
- Treatment with Metformin, Insulin Glargine (long-acting insulin) or the experimental drug Dorzagliatin which is FDA-approved for research, for up to 8 weeks
- 2-4 inpatient visits/ including two overnight stays at the Clinical Research Unit at 15th floor of Jefferson Tower.
All visits to be completed within a three-month period. Study-related tests and procedures are provided free of charge. Participants will be compensated up to $2,500 for study completion. All Participants must be Diagnosed with type 2 DM and Not on any type of insulin therapy.
Please contact Kathryn Hollifield-Laumer at khollifield@uabmc.edu or co-investigators Chanel Mason at 205-934-1921 or cnmason@uabmc.edu, or Alaaeldin Hodhod at ahodhod@uabmc.edu.







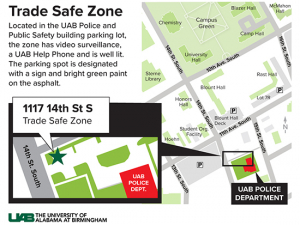
















 UAB is committed to fostering a safe and inclusive environment for all Blazers. From mobile apps to bus escort services to B-Alerts and more, make sure you’re up to date on all the ways to stay safer on campus.
UAB is committed to fostering a safe and inclusive environment for all Blazers. From mobile apps to bus escort services to B-Alerts and more, make sure you’re up to date on all the ways to stay safer on campus.