Players walk in the virtual world by moving their arms, and can observe their environment by turning their head.In 2005, a teenage Jereme Wilroy was visiting his grandmother’s house in Grand Bay, Alabama, located about two hours northeast of New Orleans, helping her recover from Hurricane Katrina, a Category 5 storm that devastated the Gulf Coast from central Florida to Texas and killed more than 1,200 people.
Players walk in the virtual world by moving their arms, and can observe their environment by turning their head.In 2005, a teenage Jereme Wilroy was visiting his grandmother’s house in Grand Bay, Alabama, located about two hours northeast of New Orleans, helping her recover from Hurricane Katrina, a Category 5 storm that devastated the Gulf Coast from central Florida to Texas and killed more than 1,200 people.
During the clean-up, Wilroy’s father cut down a damaged tree, and while Wilroy tried to direct its fall, something went wrong. He fell, and then the tree fell on top of him, breaking his fifth thoracic vertebrae along with several ribs and puncturing a lung.
Because Wilroy was injured so severely and cell service hadn’t yet been restored after the hurricane, it took hours for a helicopter rescue crew to reach him and transport him to safety.
“It’s just a miracle I lived,” he said.
Understanding the basics
Wilroy, now a postdoctoral scholar at UAB studying in the Department of Physical Medicine and Rehabilitation, said the spinal cord injury left him paralyzed below the waist. He often experiences neuropathic pain, or nerve-based sensations that can manifest themselves as pain, discomfort or, in Wilroy’s case, pins and needles in the bottoms of his feet. With time, his symptoms have shifted to “more tingling sensations, nothing super painful” in his legs, although his injury line can experience more “stabbing, shocking-type” feelings. He knows others with comparable injuries who have similar experiences.
|
“Gamification is important and allows interaction with the environment. When you have a goal, your brain lights up more.” |
Neuropathic pain is a common complication for many patients with spinal cord injury, described in 2015 as found in between 60-69 percent of that population. It’s often chronic, and doesn’t respond well to any single drug, although anti-depressants, anti-epileptics, opioids and other analgesics have been tested and often are prescribed.
“Some call it suicide pain, because it doesn’t respond well to anything — not opioids, cognitive behavioral therapy — and I’m a psychologist saying that,” said Zina Trost, Ph.D., an assistant professor in the Department of Psychology who studies clinical health and rehabilitation psychology. She focuses on factors that influence adjustment to chronic pain and traumatic injury or both.
|
“Some call neuropathic pain suicide pain, because it doesn’t respond well to anything — not opioids, cognitive behavioral therapy — and I’m a psychologist saying that.” |
In 2004, a group of scientists reported patients with phantom limb pain experienced relief from discomfort while viewing a reflected image of their intact limb in a mirror. They adapted that therapy in a test for paraplegic patients, in which their upper torso was reflected in a mirror while a video of legs walking replaced the reflection of their legs and wheelchair.
“It actually seemed to work,” Trost said, “and in this field, if anything works even a little bit, it’s great.”
Then Clinical Professor Scott Richards, Ph.D., and Assistant Professor Betsy Richardson, Ph.D., from the departments of Psychology and Physical Medicine and Rehabilitation, received a grant from the National Institutes of Health to take the concept a bit further: They created a 3-D video from a first-person point of view, then placed patients in 3-D glasses and exposed them to the videos; they observed interesting brain activation.
 Cory Shum (left) and Zina Trost, Ph.D.Building and innovating
Cory Shum (left) and Zina Trost, Ph.D.Building and innovating
When Trost joined UAB’s faculty in 2015, she set to work building on this research. She partnered with UAB’s Immersive Experience Lab (IXL) to create a virtual reality simulation that could offer the same therapeutic opportunities as mirror therapy for paraplegic patients who experience neuropathic pain.
The School of Engineering’s IXL is home to a cross-functional team of developers and artists who research the application of immersive artificial experience in learning, visualization, communication and performance, and they develop virtual reality, augmented reality and mixed-reality software and content. Trost has experience with simulations; she’s also working on a Small Business Innovation Research grant-funded project with faculty from the Department of Physical Therapy and an NIH-funded venture developing treatment for chronic low-back pain.
The theory is this, Trost says: “If [paraplegic patients] who have neuropathic pain imagine themselves doing a movement that requires their legs, they will have activation of those same networks,” providing relief from phantom pains.
|
“To actually have movement involved in it, especially to a person with a spinal cord injury, I could feel an increase in sensation. It gave me the feeling of moving around.” |
In the IXL simulation, participants sit in their wheelchairs wearing goggles and hold foot-long wands that allow scanners to track their arm movements. The goggles provide a first-person view from the perspective of their standing avatar — literally able to look down and see themselves standing — complete with a shadow that moves fluidly with the avatar. As users move their arms as one would do to walk, their avatar moves forward. Other arm motions trigger climbing and kicking movements to earn points within the game — an element Trost believes will benefit therapy.
“I come from a background of using virtual reality in a way that’s not just distracting people in order to relieve their pain,” Trost said. “Gamification is important. It allows interaction with the environment. When you have a goal, your brain lights up more.”
J.D. Rugamba, a third-year doctoral student in the Department of Mathematics, was injured in a 2014 car accident that left him paralyzed below the waist. He still experiences neuropathic pain, but says it’s become rote in the four years since the accident.
“Sometimes it gets worse, but I’m accustomed to it,” he said. “It’s different for everyone. When you have a spinal cord injury, you shouldn’t feel anything. You should feel like you’re floating, so when your neuropathic pain gets extreme, it’s bothersome.”
Rugamba, who had never played a virtual reality game or tried other treatments or medications for his neuropathic pain, was one of the earliest participants to test the IXL’s new therapy game in a small focus group. Trost says the group offered advice crucial to and irreplaceable in this kind of research: “You really can’t develop this kind of technology without the input from the folks who are going to use it. I don’t know what it’s like to experience this from their perspective.”
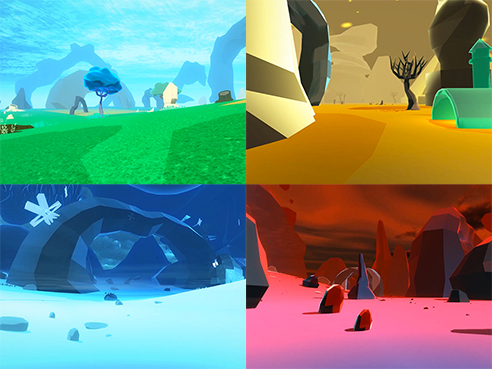 The game prototype has several environments for players to explore.
The game prototype has several environments for players to explore.
Rugamba was excited to try the game and give feedback about its potential as a therapy option.
“It has a chance — once you put the headset on you get a feeling like you’re moving. Your brain is definitely tricked. If it works, it’s definitely positive.”
Wilroy, who also was one of the focus group members, participated in an earlier version of the research elsewhere, during which he wore a headset but was only shown a prerecorded video of his avatar walking. He believes this simulation therapy has a better chance at providing relief from pain.
“I could feel an increase in sensation,” Wilroy said. “Not externally touching my legs, but it gave me the feeling of moving around.”
Recognizing the potential
Hearing positive reactions such as Rugamba’s and Wilroy’s was a career-defining moment, Trost said. Corey Shum, manager of the IXL, said the focus group participants gave constructive criticisms — “my avatar is walking into a wall,” “these legs don’t look like mine,” “I would never wear Chucks” — but the overall response was categorically positive. One participant said they felt the sensation of their hips moving.
“They were complaining about certain aspects of the thing, yet saying, ‘It felt like I was doing this,’ or ‘It felt like I was climbing,’” Shum said. “They gave me a neutral-positive response.”
One aspect both Trost and Shum found particularly interesting is that focus group participants often didn’t look at their avatar’s legs, yet still experienced sensations of movement and pain relief — a deviation from findings based on mirror therapy for phantom limb pain.“
They didn’t have to look at the legs to feel like they were walking,” Shum said. “In mirror intervention, you try to get them to look at their legs as much as possible. [In this game,] they felt swinging and moving and just being at the correct elevation was enough.”
Trost is quick to note that this therapy doesn’t mean that participants are gaining any use of their legs. What she hopes for, based on the focus group’s experiences, is a significant reduction in neuropathic pain, no matter how it varies from patient to patient.
Forming strategic partnerships
 Certain arm motions trigger climbing and kicking movements to earn points within the game.
Certain arm motions trigger climbing and kicking movements to earn points within the game.
This spring, Trost and Shum will begin a two-part study using this new virtual reality software. Two larger groups will be measured: One will employ a newer version of the interactive software that incorporates the suggestions of the focus group; a second will use only the headset and watch a prerecorded, non-interactive version of the same game.
The goal, Trost said, is to understand whether virtual reality can change brain organization. Pain psychologists believe neuropathic pain develops due to a dissonance between information entering and leaving the body. In theory, virtual reality therapy could reverse cortical changes caused by that dissonance.
Trost and Shum are pairing with a group of top brain-imagers in Australia to better understand if and how virtual reality changes the brain. Australia has a large population of people with spinal cord injuries.
“Birmingham has a specific population of people who have spinal cord injuries,” Trost said. “So as a scientist, you begin to wonder if your research only works in your specific area.”Trost and Shum are making a trade: In exchange for sharing the IXL technology with the Australian researchers, UAB will be able to access the research data generated by its use there. In addition, the Australians will train brain imagers, such as UAB Research Associate Professor Mark Bolding, Ph.D., to collect that type of data.
“We will provide the technology while they provide initial imaging and cortical analysis,” Shum said. “If we can mirror our tech out there and their imaging protocols here, there will essentially be two sides of the world doing the same thing.”
|
“If it changes things in regard to neuropathic pain, it will be successful because it will be something people want to use. Rather than have to go somewhere to work with a therapist and interact with things that aren’t necessarily enjoyable, this is fun.” |
A bright future
Ultimately, Wilroy believes the project potential is unlimited. It is pioneering and modern, and it could make therapy accessible to patients with spinal cord injuries, both financially and logistically.
“I think it’s a very innovative therapy program,” Wilroy said. “Technology is getting cheaper and cheaper. It’s becoming very affordable to have in the home.“If it changes things in regard to neuropathic pain, it will be successful because it will be something people want to use. Rather than have to go somewhere to work with a therapist and interact with things that aren’t necessarily enjoyable, this is fun.”