 During the first SARS outbreak in 2003, Kevin Harrod, Ph.D., directed experimental model testing of candidate vaccines. Within three weeks of the first cases of H1N1 swine flu appearing in the United States in 2009, "we had the virus in my lab and NIH contracts to work on research," he said. "I've been down this path before and know what it takes to get these things started."In early 2020, soon after it became clear that the COVID-19 pandemic was a serious threat to humanity, Kevin Harrod, Ph.D., UAB's resident expert on SARS viruses, got a message from Matt Might, Ph.D., director of the university's Precision Medicine Institute. "If we computationally predict drugs, can you test them?" Might wanted to know. "I said sure," Harrod said.
During the first SARS outbreak in 2003, Kevin Harrod, Ph.D., directed experimental model testing of candidate vaccines. Within three weeks of the first cases of H1N1 swine flu appearing in the United States in 2009, "we had the virus in my lab and NIH contracts to work on research," he said. "I've been down this path before and know what it takes to get these things started."In early 2020, soon after it became clear that the COVID-19 pandemic was a serious threat to humanity, Kevin Harrod, Ph.D., UAB's resident expert on SARS viruses, got a message from Matt Might, Ph.D., director of the university's Precision Medicine Institute. "If we computationally predict drugs, can you test them?" Might wanted to know. "I said sure," Harrod said.
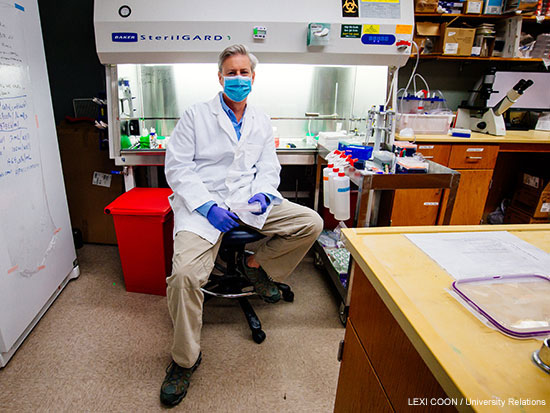
Testing drugs on SARS-CoV-2, the novel coronavirus that causes COVID-19, requires more than a few petri dishes. Harrod's lab operates at biosafety level 3, with scientists wearing full body personal protective equipment (PPE) that include their own air supplies. “Just putting it on and taking it off is an adventure in itself,” said Harrod, professor in the Department of Anesthesiology and Perioperative Medicine. “You have to be especially careful when taking it off to not touch the outside. There’s a bit of a gymnastics aspect.”
This is Harrod’s third pandemic. During the first SARS outbreak in 2003, Harrod directed experimental model testing of candidate vaccines. Within three weeks of the first cases of H1N1 swine flu appearing in the United States in 2009, "we had the virus in my lab and NIH contracts to work on research," he said. "I've been down this path before and know what it takes to get these things started."
 Ritesh Sevalkar, Ph.D., and Harrod discuss next steps for sequencing the SARS-CoV-2 virus. "There has been a great deal of camaraderie and collaboration at the School of Medicine," Harrod said. "Everyone is bringing their level of expertise to bear. That is one ancillary benefit of these tough times."
Ritesh Sevalkar, Ph.D., and Harrod discuss next steps for sequencing the SARS-CoV-2 virus. "There has been a great deal of camaraderie and collaboration at the School of Medicine," Harrod said. "Everyone is bringing their level of expertise to bear. That is one ancillary benefit of these tough times."
That is why Harrod received one of 14 pilot grants from the $1.1 million Urgent COVID-19 Clinical Research and Laboratory Research Fund, established by UAB through philanthropic giving and funding from Might's Precision Medicine Institute. But the PMI is providing more than money, Harrod noted. "This grant is really built on a team of computationalists who use artificial intelligence approaches to identify drugs that are already FDA-approved and can be repurposed,” he said.
| "This grant is really built on a team of computationalists who use artificial intelligence approaches to identify drugs that are already FDA-approved and can be repurposed.” |
"Scientists will probably figure out whole new drugs to treat COVID-19, but that will take years. This pandemic will probably be gone by then. We said, What can we do now?"
Harrod provides the biological validation of the drug candidates identified by the team from PMI. Before researchers can figure out how to kill SARS-CoV-2, Harrod and his team had to figure out how to grow it. "Some viruses are hard to grow in the lab, but we had our practice with the first SARS 17 years ago,” he said. “We used many of the same methods and lo and behold they worked well." The onset of the COVID-19 pandemic caught Harrod between grants as he was building up to work on influenza. Adrie Steyn, Ph.D., professor in the Department of Microbiology, has a team working on tuberculosis, which also requires biosafety level 3 precautions. "He said, if you need people, my team is ready to help," Harrod said. "There has been a great deal of camaraderie and collaboration at the School of Medicine. Everyone is bringing their level of expertise to bear. That is one ancillary benefit of these tough times."
 Harrod uses an inverted phase contrast microscope to examine Vero E6 cells that will be used in SARS-CoV-2 drug screening.
Harrod uses an inverted phase contrast microscope to examine Vero E6 cells that will be used in SARS-CoV-2 drug screening.
In addition to supporting UAB research efforts, Harrod's lab is serving the Biomedical Advanced Research and Development Authority (BARDA), which is the federal agency charged with developing countermeasures against COVID-19. "We're providing the virology support," Harrod said. "We grow the viruses, measure them and provide them to the BARDA contractor."
Harrod has already begun to unleash compounds identified by PMI on viral cultures in the lab. "We have a three-tier screen that we work through," moving through a range of cell types, ending with human lung cells, he said. Steven Rowe, M.D., professor and director of the Cystic Fibrosis Research Center at UAB, is working to develop animal models of COVID-19, something he has pioneered for cystic fibrosis. Rowe’s team is also growing the human lung cells that Harrod tests in his biocontainment lab.

"We have as many as 15 drugs that have been identified and there are going to be candidates that are worthy of consideration" for human testing, Harrod said. "The beauty is these are all FDA-approved drugs so they could be mobilized really quickly."
Read more on UAB's urgent COVID-19 research projects

Glow-in-the-dark coronavirus testing is first project of new hire

Researchers model COVID-19 infection in 3D human lung tissue

Researchers are creating a coronavirus showdown to settle pressing antibody problems

Where are the good antibodies against COVID-19? UAB project aims to find out
Helpful viruses may unlock the secrets of coronavirus antibodies
Cytokine storm treatment for coronavirus patients is focus of first-in-US study