Earlier in his career, David Askenazi, M.D., MPH, heard a colleague speak about how important medical innovation is and how academics should devote time and effort to inventing devices and products to aid patients’ health, rather than only focusing on observing and reporting.
“He said that in order to change the course of those we encounter in our daily rounds, we must bring innovations to the bedside,” Askenazi said. “After all, we have a front-row seat in understanding where the gaps in optimal care exist.”
That message stayed with Askenazi as he carried out his duties as the Medical Director of the Pediatric and Infant Center for Acute Nephrology (PICAN) at Children’s of Alabama and as a Professor and the W. Charles Mayer Endowed Chair in Pediatric Nephrology at UAB.
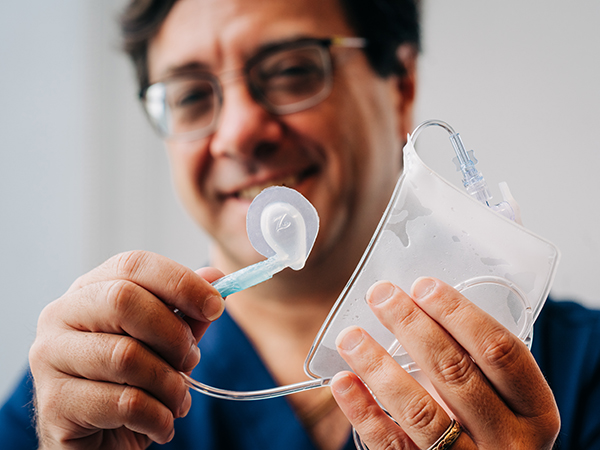 David Askenazi holds the Zorro-Flow device, which he designed with the help of a team from UAB and Children's of Alabama.
David Askenazi holds the Zorro-Flow device, which he designed with the help of a team from UAB and Children's of Alabama.
“In both the clinical and research arena, I found myself continually asking, ‘Why doesn’t someone make a safe and effective urine collection device for neonates?’ until one day it hit me: Why can’t we do it here in Birmingham?” he said.
Askenazi gathered a business team, including James Wilkie and Bruce and Eva Ovitt. Wilkie has 35 years of experience in the life sciences industry developing both drugs and devices. Bruce and Eva Ovitt collectively bring a complementary set of skills and experience in various healthcare business startups, branding, marketing, and sales. Askenazi brought Wilkie and the Ovitts into the fold, and the UAB Harbert Institute for Innovation and Entrepreneurship (HIIE) assisted them in disseminating management duties.
To bring Zorro-Flow from paper sketches to a physical device, the team enlisted the help of Martin Holland, then a graduate student in the UAB School of Engineering. Two nurses from Children’s, Elizabeth Dechant and Shelby Leverett, also advised on the design of the device. As ostomy nurses, they provided valuable insight on how to adapt the device to provide the most comfort for young patients.
In July 2022, Askenazi’s team launched Zorro-Flow, the newest start-up with the HIIE. Zorro-Flow is a neonatal external urine-collection device, which has been designed to collect urine safely and effectively in critically ill female neonates and children.
Collection and recognition of whether urine is being produced is essential, but current urine-collection methods used in neonates are often ineffective. Catherization is difficult in neonates, and collection bags or diaper weight measurements are not accurate and can cause discomfort. Due to the lack of accurate tools, current clinical practices vary drastically among institutions.
“In every critically ill patient at our institution, we can reliably collect urine. The exception happens to be some of the most vulnerable patients: premature neonates and children,” Askenazi said. “As a world leader in the field of neonatal nephrology, I am convinced that providers will care for these vulnerable children better if we have the right tool.”
Askenazi said he feels enormous gratitude to all of the people who had a role in getting the company off the ground. He is optimistic about the path ahead.
“There’s a tremendous amount of excitement in the field as we communicate our goal to bring this device to the bedside in 2023,” he said. “There is a bit of nervous energy considering the huge learning curve that comes with starting a company.”
The HIIE was integral to Zorro-Flow’s success, helping give Askenazi a basic foundation in entrepreneurship.
 The Zorro-Flow device is designed to safely and comfortably collect urine from neonates.
The Zorro-Flow device is designed to safely and comfortably collect urine from neonates.
“The Harbert Institute team provided me with a framework to work through and helped guide me through the multiple processes of forming a team, developing a plan, applying for internal seed funds, and the multiple steps needed to file an international patent,” Askenazi said. “I learned that you have to begin with the end in mind, the importance of gaining and keeping momentum, and the importance of finding great people to help you through the process.”
Within the next year, Askenazi hopes to file the female version of Zorro-Flow as an FDA class 2 device, which will require manufacturing systems, regulatory documents, and quality evaluations. Such evaluations have already begun, he said. He also hopes to solidify the business framework, complete the Series A funding round and launch the devices for beta testing at several large academic centers focused on neonatal nephrology.
Within three years, Askenazi looks to complete the Series B funding round, file the male version as an FDA class 2 device and scale up the manufacturing of the Zorro-Flow devices.
Askenazi said he hopes his fellow UAB faculty and staff will re-examine their areas of expertise to envision improvements for patients.
“If you see a gap in your ability to care for your patients, spend some time thinking about how innovation can help you better care for your patients. UAB has so many talented individuals on campus, there is likely someone out there that can help you find solutions,” he said. “Speak to people in the HIIE and around campus who understand patents, commercialization, and the business of devices and drugs.”
In 2020, the HIIE split $300,000 among 11 UAB early-stage projects – including Zorro-Flow – as part of the U.S. Economic Development Administration’s Regional Innovation Strategies program, now known as the Build to Scale program. The initial pilot funding for Zorro-Flow prototypes came from the Tolwani Innovation Fund in Nephrology.