In fiscal year 2019, UAB researchers received a record $602 million in grants and contracts, marking a second year of double-digit percentage growth in funding. That productivity has carried on unabated in 2020, and you can follow along. Every Tuesday, Research Administration releases a list of the grants and contracts awarded the previous week (BlazerID required).
In this new series, we’re spotlighting new or re-funded projects to offer a window into the groundbreaking, lifesaving work done by our colleagues around campus. (See the first two stories, profiling grants to stop online opioid sales and to build freezers for the International Space Station.)
This week, we’re taking a look at a new strategy to tackle the autoimmune disease lupus.
Project title: Cytokine Immunoregulatory Strategy to Target SLE Autoreactive B Cells
Principal investigator: Hui-Chen Hsu, Ph.D., associate professor, Division of Clinical Immunology and Rheumatology; co-investigator: John Mountz, M.D., Ph.D., professor, Division of Clinical Immunology and Rheumatology
Funding: Three years, $597,000 from the Lupus Research Alliance
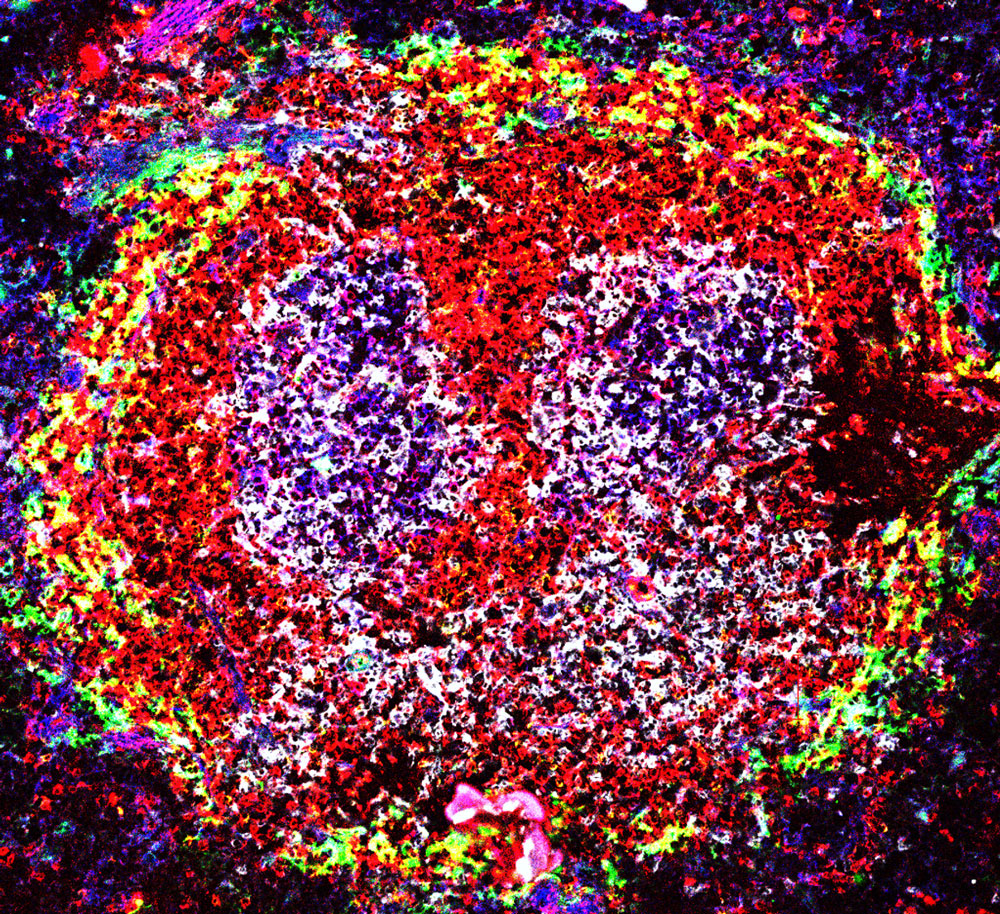 Spontaneous germinal centers (the two purple structures) in the spleen of a lupus-prone BXD2 mouse. Image courtesy Hui-Chen Hsu.
Spontaneous germinal centers (the two purple structures) in the spleen of a lupus-prone BXD2 mouse. Image courtesy Hui-Chen Hsu.
Lupus is an autoimmune disease marked by hyperactive B cells. These immune cells normally produce antibodies that recognize invading bacteria and viruses. But in lupus they mistakenly attack the body’s own healthy tissue, causing swollen joints, rashes, kidney disease and other symptoms. According to the Lupus Research Alliance, the leading private funder of lupus research, the disease is most often diagnosed in young women ages 14-44 (nine out of 10 adults with the disease are women; it is more common in women of African American, Hispanic, Asian and Native American descent, compared with Caucasian women).
One of the hottest topics in lupus research is the search for molecular factors that allow rogue, self-reactive B cells to escape the safety checkpoints involved in the complicated process of B cell development. A particularly interesting case are the mysterious, so-called double-negative B cells, which do not fall into any of the common subtypes of adult B cells and are a hallmark of patients with lupus and other autoimmune conditions.
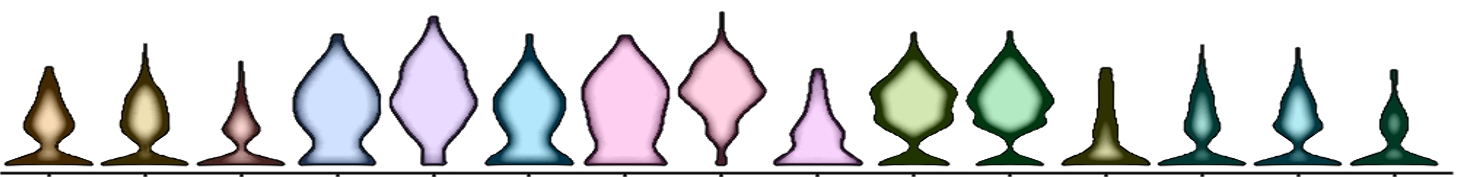 A violin plot based on single-cell sequencing; each tree represents the gene expression result of a specific subset of B cells. Image courtesy Hui-Chen Hsu.
A violin plot based on single-cell sequencing; each tree represents the gene expression result of a specific subset of B cells. Image courtesy Hui-Chen Hsu.
For several years, physicians in UAB’s lupus clinics have partnered with immunologists to get a better understanding of the differences between B cells from patients with lupus and B cells from non-affected individuals, said Hui-Chen Hsu, Ph.D., who is principal investigator on a grant from the Lupus Research Alliance that is exploring a new therapeutic strategy against lupus. Winn Chatham, M.D., clinical director for lupus clinics at UAB and professor in the Division of Clinical Immunology and Rheumatology, has been a critical collaborator, Hsu said. “He sees more than 500 lupus patients here at UAB and has formed a very strong collaborative pipeline to recruit patients and collect samples,” she explained. “He’s not just helping me, but everyone who studies lupus at UAB.”
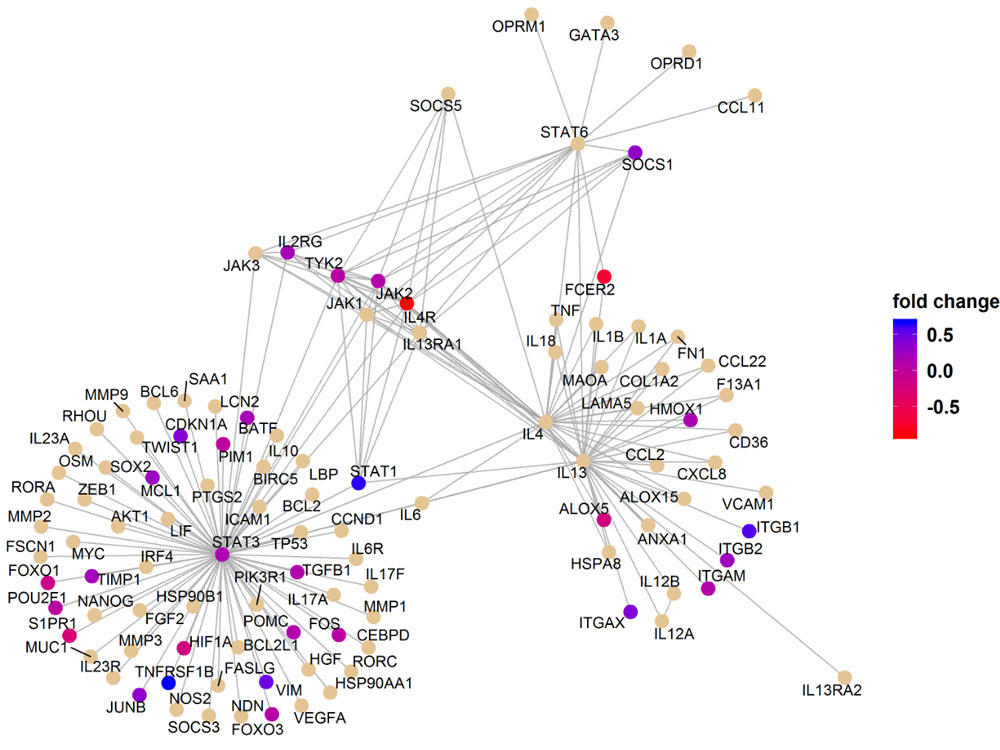 A reactome pathway analysis from Dr. Hsu's research. Image courtesy Hui-Chen Hsu.
A reactome pathway analysis from Dr. Hsu's research. Image courtesy Hui-Chen Hsu.
Using single-cell RNA sequencing on those patient samples, which allows researchers to zoom in on previously unknown cell subpopulations, Hsu and Mountz made a “very interesting discovery,” Hsu said. (Read more about single-cell RNA sequencing capabilities at UAB here.)
In a pre-clinical laboratory test, when they gave B cells from lupus patients a specific combination of two interleukins — signaling molecules important in lupus — “the B cells were arrested in a resting naïve state and they did not develop into this pathogenic double-negative state,” Hsu said. “When I first described the data in a lupus meeting, the idea seemed to be perplexing.” But after seeing her evidence, the Lupus Research Alliance gave her the opportunity “to determine if the proposed combined therapy could be the missing piece of the treatment puzzle that can potentially help many patients,” she said. “I am grateful for their support.”
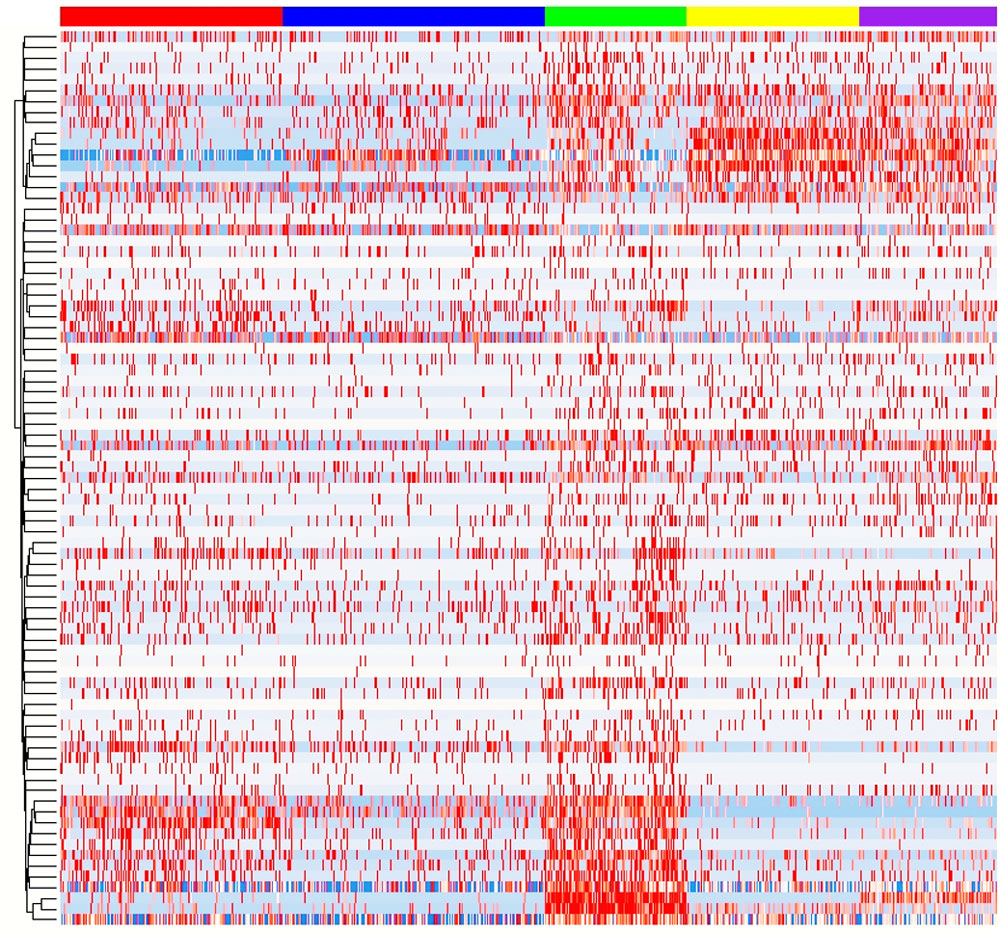 Heatmap clustering of differentially expressed genes in B cells of SLE versus healthy individuals. Image courtesy Hui-Chen Hsu.
Heatmap clustering of differentially expressed genes in B cells of SLE versus healthy individuals. Image courtesy Hui-Chen Hsu.
Hsu and Mountz also found lower levels of IL-4 receptors on the surfaces of B cells from lupus patients, suggesting that this could be another signature of disease in these patients and may be an indicator of increased disease severity. As part of their grant from the Lupus Research Alliance, the researchers will also work to develop a lupus mouse model to investigate these effects more extensively than they can in human cells.
“The preliminary data is very encouraging,” Hsu said. “If we can maintain B cells at this resting naïve stage it could prevent recurrence of flares in patients and offer a new therapy to treat the disease.”
Find out more about single-cell sequencing research at UAB — and read the first two stories in our Discoveries on Deck series
Symphony for a single cell: sequencing and time travel at UAB

Discoveries on deck: Blocking fentanyl at the source