-
Project to predict and prevent apnea in premature babies receives Blazer Bridge Fund supportAfter studying thousands of hours of data from heart rate monitors, UAB clinician-researchers developed algorithms to predict when a baby’s heart rate will drop to unsafe levels in the NICU. With funding from UAB’s Harbert Institute, they will now conduct a clinical trial of a device they have created to intervene automatically.posted 10 days ago 258 viewsHealth Services Foundation awards $1.5 million in General Endowment Fund grants for 2024A 3D printer for prosthetics, navigation for patients on dialysis and a new electron microscope are three of the 11 projects to receive funding. The HSF-GEF faculty grants program is a way for UAB to support innovative ideas from its researchers and clinicians.posted 11 days ago 927 viewsUAB Comprehensive Diabetes Center starting denosumab trial in adults with established Type 1 diabetesThe Denosumab for Type 1 Diabetes phase 1/2 clinical trial, is a collaborative study with the Arthur Riggs Diabetes and Metabolism Research Institute and the Center for Diabetes and Metabolic Diseases at Indiana University.posted a while back 1486 viewsUAB’s Research MRI Core specializes in insights into the brain and bodyFrom a 3 Tesla PET/MR to a 0.55 Tesla Freemax, this core facility offers research tools that are crucial to UAB’s neuroscience studies and more, as well as expert advice on the best protocols to use for individual experiments.posted a while back 895 viewsMost teens with autism do not drive. This researcher is testing a way to change that.Emma Sartin, Ph.D., MPH, assistant professor in the Department of Health Policy and Organization at the School of Public Health, is leading a Department of Defense-funded project that is developing a virtual assessment to help autistic individuals and their parents decide whether they are ready to drive.posted a while back 2293 viewsUAB’s first NIH R38 StARR grant helps more doctors get involved with research during residency
The Stimulating Access to Research in Residency program, funded by the National Institute of Allergy and Infectious Diseases, is designed to help address the shortage of physician-scientists nationwide.
posted a while back 1429 viewsMaking sense of the data deluge with the UAB Biological Data Science coreU-BDS specializes in analyzing genomic and transcriptomic data, creating data pipelines, and keeping up with the latest methods and algorithms required for cutting-edge research.
posted a while back 2205 viewsInternational recognition highlights the value of UAB’s cutting-edge Flow Cytometry and Single Cell CoreThe facility, one of 15 shared resource labs at UAB and among the busiest, is one of a handful of leading labs to be recognized by the International Society for Advancement of Cytometry, or ISAC.
posted a while back 1728 viewsMeet the leader of UAB’s Climate and Health InitiativeZhen Cong, Ph.D., professor in the Department of Environmental Health Sciences in the School of Public Health, shares her work on disaster preparedness of older adults and why she wants all Blazers to take part in the initiative’s work.posted a while back 1800 viewsDoes your body really fight against weight loss? This scientist explains why the research says no.Many people, including clinicians and researchers, think “the main reason people regain weight after weight loss is because the body fights back” in a phenomenon called metabolic adaptation, said UAB researcher Cátia Martins, Ph.D. Martins, a leading scientist studying metabolic adaptation, explains what she has found and her plans for a groundbreaking clinical trial.posted a while back 9408 viewsThis neuroscientist is starting seizures in order to stop themRachel June Smith, Ph.D., a key recruit in UAB’s Neuroengineering and Brain-Computer Interface Initiative, can predict the frequency of stimulation that will push a brain into the chaos of a seizure — potentially saving patients with intractable seizures time, frustration and money.posted a while back 3736 viewsHow the HSF’s General Endowment Fund awards help UAB compete on a national stageOver its 28 years, this signature program has awarded grants totaling almost $60 million. Meet recipients and see how the HSF-GEF grants make UAB “a place where, if you have a good idea, you can find the support to make it a reality.”posted a while back 3757 viewsLargest whole-genome sequencing study in Kawasaki disease could point to precision treatmentResearch by UAB genetic epidemiologist Sadeep Shrestha, Ph.D., sheds light on a mysterious condition that is now the leading cause of acquired heart disease among children in the United States.posted a while back 3563 views4 cutting-edge machines powering UAB discoveriesWith research awards breaking all-time records, we toured labs where high-tech tools are driving science forward.posted a while back 4311 viewsMeet the UAB doctors protecting Alabama from killer fungiAlabama is a hotbed for fungal diseases — which is why experts in treating and tracking problematic fungi gravitate to UAB. This is great news for Alabamians as killer fungi become a worldwide threat.
posted a while back 8541 viewsFive lessons from the first five years of the All of Us Research ProgramHow the ambitious NIH initiative is turning precision medicine dreams into reality for hundreds of thousands of Americans left behind by previous studies — and where it is going next.posted a while back 6995 viewsFatal crashes rose in Alabama over past 3 years, new UAB study findsDespite fewer drivers on Alabama roadways and a decline in injuries per accident, fatal crashes rose over the three years from 2020 through 2022, according to a new analysis by UAB researchers published in the journal Accident Analysis and Prevention.posted a while back 3730 viewsAmid global ChatGPT obsession, UAB conference examines AI’s role in advancing health care and researchExperts at ATTIS 2023 shared reports from the front lines, including how they are using ChatGPT in their labs, the need for regulation and why this is a “tremendous time” for health care.posted a while back 4683 viewsA neonatologist chooses to become a machine-learning expert to improve patient outcomesWith data from 25,000 deliveries at UAB, Vivek Shukla, M.D., aims to predict which fetal heart rates are cause for concern. He is also earning a Ph.D. in engineering to bridge the gap between clinicians and data scientists.posted a while back 3994 views“Dr. Impossible” aims to bring brain tech to the peopleTake a trip into the Alabama BRAIN Lab in UAB’s Spain Rehabilitation Center, where a team led by neuroengineer Jamie Tyler, Ph.D., is working with patient groups to test promising neuromodulation treatments for chronic pain, insomnia and more.posted a while back 8790 viewsCollat Professional Ed partners with researchers on leadership and professional development academyThe goal is to offer career development opportunities for all members of the NSF-funded IISAGE grant, led by Associate Professor Nicole Riddle, Ph.D., in the Department of Biology.
posted a while back 2988 viewsWhy do super-agers skew female? These researchers are drilling into the hottest hypotheses.In a search that encompasses geckos, bats, sex-switching fish and more, the NSF-funded IISAGE team is seeking data to explain lopsided lifespans. A key question: How much wiggle room is there in aging?posted a while back 5212 viewsThis med student is on a mission to make tattoo inks safeA chemical mystery drew Matthew Kiszla into tattoo research: Why are red inks most likely to cause rashes and other reactions? Now he is working to analyze commercial inks and looking for collaborators both scientific and artistic.
posted a while back 15013 viewsInnovation Institute hosts country’s first metahealth symposium, Feb. 28The event will explore a future of health care that “is going to be anchored in the metaverse” and be the first such symposium to be held both live and in the metaverse itself, according to Rubin Pillay, M.D., Ph.D., executive director of the Marnix E. Heersink Institute for Biomedical Innovation.posted a while back 4030 viewsFour high-tech health devices being tested at UAB nowTake a look at new technologies being studied at UAB for treatment of depression, sleep apnea, traumatic brain injury and tic disorders.
posted a while back 6006 views3 UAB chemists break down their formulas for fighting cancerNew treatments emerging from the labs in the Department of Chemistry rely on split-second timing, tiny cargo bubbles and supercomputer-powered predictions.posted a while back 5583 viewsWhat you need to know about the new NIH data management and sharing policyBeginning Jan. 25, 2023, the National Institutes of Health will implement a new data management and sharing policy, which will increase the rigor, reproducibility and transparency of research and create open access to data.
posted a while back 4957 viewsMajor NIAID grant brings cutting-edge equipment to UAB for research on COVID and moreUAB scientists will have a new arsenal of state-of-the-art, high-end technology for their investigations in infectious diseases and pandemic preparedness through a $4.3 million scientific equipment grant from the National Institute of Allergy and Infectious Diseases.
posted a while back 3915 viewsResearchers hack adaptive cruise control, then show how to make it saferDriver assistance tech that comes standard on new vehicles can be tricked into causing accidents — but there is a way to alert humans in time. A UAB grad student and his mentor will share their findings this month at a global conference.
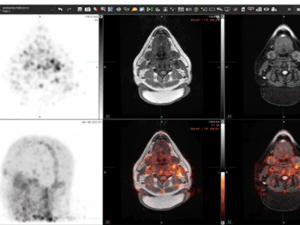
posted a while back 7856 viewsFirst-of-its-kind scan tracks immune invaders to explore puzzling brain diseasesPatients with multiple sclerosis, fibromyalgia and chronic fatigue syndrome will soon be able to enroll in the clinical trial of a PET agent that can capture evidence of brain infiltration by white blood cells and could eventually guide treatment.posted a while back 6031 viewsSalt boosts blood pressure for some people. UAB study asks: Who?By alternating high-salt and low-salt diets, a new clinical trial aims to find out how common salt sensitivity of blood pressure is in the general population. The researchers are also exploring whether the immune system plays a role.posted a while back 15337 viewsStudy: Expert-led discussions change student perceptions of COVID vaccinesAfter undergraduates in introductory biology courses talked with an epidemiologist and a physician specializing in infectious diseases, 60% who initially said they would not get vaccinated had changed their minds.posted a while back 5507 viewsMore faculty share the stories behind their development grantsPlant-based diets, biased language in the courts and the trouble with night lights: Recipients of 2022 Faculty Development Grant Program awards explain how they will use their funds.posted a while back 4964 viewsApply for fellowship to explore careers in technology transfer, commercializationGraduate students and postdoctoral fellows in the Innovate Fellows program are trained and compensated to evaluate new inventions on campus through market, prior art and patent analyses to assess commercial merit.
posted a while back 5420 viewsFour faculty share the stories behind their development grantsA civil rights field experience, safer MRI scans, investigating college stress and implementing a massive genetic test for cancer: Recipients of 2022 Faculty Development Grant Program awards explain how they will use their funds.
posted a while back 6106 views22 faculty receive grants to fund developmental projectsThe UAB Faculty Development Grant Program supports junior faculty with funding to pursue research, creative works and scholarly activity.
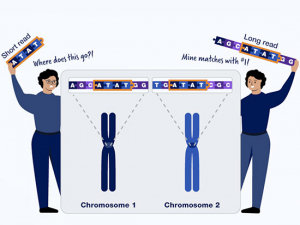
posted a while back 8757 viewsResearchers pioneering long-read sequencing studies explain why long reads matterNew technologies are filling in gaps in the human genome and opening major areas for discovery. Zechen Chong, Ph.D., and Robert Kimberly, M.D., explain the pros and cons and how they are using long reads at UAB.posted a while back 8851 viewsDespite gains in bone marrow transplant survival, late mortality still a concern, study findsResearch led by UAB’s Institute for Cancer Outcomes and Survivorship finds that patients who received BMT using their own cells over the past three decades lived on average seven years fewer than peers, but newer strategies have narrowed the mortality gap.posted a while back 37558 views‘Motor-gaming’ stroke tele-therapy matches in-person results at much lower cost, study findsA “flipped” approach to therapy using video games and short, motivational telehealth visits could spread the benefits of stroke rehabilitation far more widely.posted a while back 6273 viewsWhy do students choose immunology? New study answers questions on a crucial pipelineResearchers explore how to help budding scientists fall in love with a field that is incredibly important but can be “very overwhelming” to start.posted a while back 9589 viewsUpcoming Events
More Events